Narrowly dispersed imprinted microspheres with hydrophilic polymer brushes for the selective removal of sulfamethazine†
Abstract
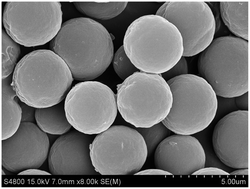
In this work, a facile and general protocol to synthesize molecularly imprinted microspheres (MIMs), combining reverse atom transfer radical polymerization with precipitation polymerization, is described. Well-tuned surface properties in the as-obtained microspheres were achieved through the “living” nature of the active ATRP-initiators present on the surface in order to further functionalize the microspheres with hydrophilic poly(2-hydroxyethyl methacrylate) (PHEMA) brushes. The presence of a PHEMA shell on the MIMs was confirmed by SEM, FT-IR and water contact angle studies, and some quantitative information is also provided. Batch adsorption experiments were carried out in pure water to investigate the equilibrium, kinetics and selectivity properties of the imprinted materials. The adsorption data of the ungrafted imprinted materials for sulfamethazine (SMZ) were fitted well to the Freundlich isotherm model. However, after grafting, the Langmuir isotherm fits the data better, mainly because of the changes to the surface properties that increase the hydrophilicity and suppress the hydrophobically driven non-specific interactions. The imprinted microspheres exhibit a large adsorption capacity, and are also specific to SMZ but non-specific to other antibiotics. The obtained materials also show rapid kinetics and great reusability and stability, displaying potential for practical applications for the selective removal of pollutants from aqueous environments.

 Please wait while we load your content...
Please wait while we load your content...