Gas-injection of gold nanoparticles and anti-oxidants promotes diabetic wound healing
Yi-Huei Huang†
a,
Chao-Yi Chen†bc,
Po-Jung Chenbc,
Shan-Wen Tana,
Chia-Nan Chena,
Han-Min Chenbc,
Chi-Shun Tuc and
Yao-Jen Liang*bcd
aGold NanoTech Inc., Taipei, Taiwan
bGraduate Institute of Applied Science and Engineering, Fu-Jen Catholic University, New Taipei City, Taiwan
cDepartment and Institute of Life Science, Fu-Jen Catholic University, No. 510 Zhongzheng Road, Xinzhuang Dist, New Taipei City 24205, Taiwan, ROC. E-mail: 071558@mail.fju.edu.tw; Fax: +886-2-2905-2193; Tel: +886-2-2905-2468
dBiomedical and photonic interdisciplinary research center, Fu-Jen Catholic University, New Taipei City, Taiwan
First published on 4th November 2013
Abstract
Diabetic ulcers and unhealed bedsores have resulted in serious complications all around the world. Anti-oxidant epigallocatechin gallate (EGCG) has been proved beneficial in diabetic studies. However, the low bioavailability of EGCG is always a problem for human application. In this study, topical gas-injection of a EGCG and gold nanoparticle (AuNP) liquid mixture (AuE) using the GNT GoldMed™ Liquid Drug Delivery System significantly accelerated the wound healing on wild-type and streptozotocin-induced diabetic mouse skin. Immunoblotting of the diabetic wound tissue showed a significant increase of the vascular endothelial cell growth factor on day 7 and the Cu/Zn superoxide dismutase expression from day 3 to day 7. Furthermore, the epidermal growth factor receptor and collagen I & III protein expression both increased significantly in the wound area. After gas-injection of the AuE liquid, hyaluronic acid (HA) expression also significantly increased on day 7 as measured by immunohistochemistry analysis. In conclusion, gas-injection of AuE significantly increases the rate of wound healing both in wild-type and diabetic mice. This study may provide a new approach for improving the EGCG bioavailability on diabetic wounds.
1. Introduction
Nanotechnology has been studied for many years and some techniques involving nanotechnology have been approved for clinical use with humans. The first micellar drug Sandimmune® was approved by the US FDA in 1983.1 Nano-materials are different from traditional materials and may have unique and otherwise unavailable biological properties, which make them suitable for use in diagnostic detection and treatment purposes.2,3 In addition, nano-materials can interact with cellular proteins and organelles, which make them suitable for the development of new medical and pharmaceutical materials. The global market share for biomedical nanotechnologies is expected to grow to US $70-160 billion by 2015, potentially rivalling the current worldwide market for biologics.4 The physical and chemical properties of gold nanoparticles (AuNPs) are different from those of other materials, which make them suitable for wide use in detection5 as well as drug delivery.6 AuNPs provide a highly multifunctional platform with which to sensitize cells and tissues for treatment regimens,7 to monitor and guide surgical procedures,8 and to preferentially administer electromagnetic radiation to disease sites.9 When transported into the bodies of animals or humans, they can resist enzyme decomposition. AuNPs can be routinely surface functionalized with active ligands at densities (1.0 × 106 μm−2)10 that are 100- and 1000-fold higher than what it is achievable with conventional liposomes11 or poly(lactic-co-glycolicacid) nanoparticles,12 respectively. Because of their comparability in size with the distances between cell-surface targets, Au nanostructures can simultaneously engage multiple, adjacent receptor sites, achieving increased selectivity in their uptake through this multivalent avidity.13 This new treatment strategy promises a potentially more efficacious approach. Diabetic ulcers are a common problem that in many cases result in amputation of the limbs in diabetic patients and severely affect their quality of life. New methods for the treatment of ulcer wounds can be developed using nanoparticles.Green tea extract epigallocatechin gallate (EGCG) is extracted from dry unfermented tea leaves and has been consumed by humans for over a thousand years. Recent research has shown that drinking tea provides health benefits, including the reduction of the likelihood of the patient developing cardiovascular diseases and cancer. Drinking tea can also reduce inflammation as well as provide resistance against infections from bacteria and viruses.14–16 However, when these results, which have been confirmed through cellular and animal experiments, are applied to humans, a problem of low bioavailability arises.17 The main cause of this low bioavailability is that green tea extracts are unstable in alkaline or neutral environments,18 and their high solubility in water makes it difficult for these extracts to pass through cell membranes. Each kilogram of orally ingested green tea contains only 20 mg of green tea extract, but only 78 ng can be detected in the blood stream, which is much lower than the concentration required for cellular experiments.19 Furthermore, bioavailability is also determined by the delivery method as well as it limits the types of organs on which the substance can be applied. Therefore, this study presents a gas-injection GNT GoldMed™ Liquid Drug Delivery System to increase the bioavailability of green tea extracts in diabetic mice, in an effort to treat wounds on the surface of the body that do not heal easily. We suggest that gas-injection with gold nanoparticles may protect the EGCG biological activity in deep cutaneous areas without it being degraded by environmental enzymes.
2. Experimental
2.1 Preparation and characterization of AuNPs
AuNPs provided from Gold NanoTech Inc (Taipei, Taiwan) were prepared by a proprietary molecular beam epitaxy process as follows. AuNPs were produced by physical manufacturing and did not contain any surface modifiers or stabilizers. Briefly, the gold bulk material was cut or ground into the target material. Then the Au target was vaporized to the atomic level by an electrically gasified method under vacuum. The vapor was condensed in the presence of an inert gas and then aggregated to form AuNPs. The AuNP sizes can be effectively tuned depending on the evaporation time and electric current used. The AuNPs were collected in a cold trap and centrifuged to obtain the final product in sterile water. The initial concentration of these AuNPs was determined by an inductively coupled plasma mass spectrometer (ICP-MS, PE-SCIEX ELAN 6100 DRC, Waltham, MA, USA). The size of the various AuNPs was measured by JEOL JEM-1200 transmission electron microscopy (TEM) (Tokyo, Japan) operated at 110 kV. The size distribution of the AuNPs in sterile water was computed with a software based on more than 100 particles in the images. The UV-Vis absorbance of AuNPs with various sizes was measured by UV-Vis spectroscopy (Hitachi U-2000, Tokyo, Japan). The negative surface charge of the AuNPs was determined by the zeta potential (Zetasizer nano-zs, Malvern, Worcestershire, UK). The stability of the AuNPs in the experiment was examined by TEM. The diameter of the utilized AuNPs was found to be 3–5 nm.2.2 GNT GoldMed™ Liquid Drug Delivery System
This hand-held, easy-to-operate drug delivery device uses low-pressure gas (N2) as the propulsion power source, and its unique gas–liquid mixing atomizer ensures high delivery efficiency. It allows the utilization of external portable gas cylinders for a superior mobility. The time and pressure parameters of the device can be set independently. The needless syringe with the liquid treatment is inserted into the device specific cassette and the cutaneous wound area is moved directly below the operating tube. After choosing the distance, the switch bottom on the holder is turned on to complete the painless drug delivery operation.2.3 Diabetic full-thickness wounds and wound measurement
Male BALB/c mice were injected with streptozotocin (STZ, 250 mg kg−1, intraperitoneal) in citrate buffer (pH = 4.5). Blood glucose levels were determined 7 days after the STZ injection and only mice with blood glucose concentrations more than 16 mmol l−1 were used in the following study. All mice were maintained on a standard laboratory diet and water ad libitum. All mice were used experimentally when they were 8 weeks old and weighted 20 g at the time of wounding. Mice were anesthetized using 2 to 2.5% vaporized inhaled isoflurane and the dorsal skin was cleansed with Betadine. Under sterile conditions, the dorsal area was completelly shaved and a single full-thickness excisional linear wound (1 cm) was created on the bilateral upper back of each diabetic mouse using a sharp scissor and a scalpel. The left wound served as the vehicle control (vehicle treatment) and the right wound was treated with 1 mg g−1 EGCG (E), 30 mg g−1 alpha lipoic acid (ALA), 0.07 mg g−1 AuNP or AuE (0.07 mg g−1 AuNP + 30 mg g−1 EGCG) liquid by gas-injection applied directly to the wound site daily in a blind manner. For the AuE preparation, EGCG powder was dissolved in PBS and a AuNP solution was added in a specific concentration.In order to determine the different healing efficiencies, the residual wound size was measured from the unclosed wound area after 1, 3, 5 and 7 days using the digital Dino calculation software (AM3013T Dino-Lite Premier, AnMo Electronics Corp., TW). Six mice in each group were euthanized on days 3, 5 and 7 post wounding and skin tissue samples from the wound areas were excised in full depth and bisected from all of the mice for biochemical analyses or H&E histological staining. This investigation conforms to the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health and the Animal Care Committee of Institute for Frontier Medical Sciences, Fu-Jen University (A9658).
2.4 Western blot analysis
Total protein samples were mixed with sample buffer, boiled for 10 min, separated by 10% SDS-PAGE under denaturing conditions, and electroblotted to nitrocellulose membranes (Amersham Pharmacia Biotech, CB, UK). The nitrocellulose membranes were blocked in blocking buffer, incubated with mouse anti-collagen I & III (Abcam plc, MA, UK), anti-superoxide dismutase (SOD-1) and anti-vascular endothelial growth factor (VEGF) (Santa Cruz Biotechnology Inc., CA, USA) antibody, washed, and incubated with horseradish peroxidase-conjugated secondary antibodies. Signals were visualized by enhanced chemiluminescent detection.2.5 Immunohistochemistry
Formalin-fixed paraffin-embedded skin tissues were analyzed. 4 μm sections were deparaffinized, rehydrated, and washed with TBS/Tween-20. Antigens were retrieved by exposure to 1 mM EDTA (pH 8.0; DakoCytomation) for 20 minutes. The endogenous peroxidase activity in the samples was blocked by exposure to 3% hydrogen peroxide–PBS and serum-free protein block (DakoCytomation). Tissue sections were incubated with primary antibodies overnight at 4 °C. Standard avidin/biotin immunoperoxidase methods with diaminobenzidines as the chromogen were used for detection (DakoCytomation).2.6 Statistical analysis
The data are expressed as mean ± S.E.M (standard error of the mean). A student's t-test was used for comparison of the parametric variables between the two groups, while ANOVA with a repeating measurement design was used for time course changes. Statistical significance was evaluated by the Tukey–Kramer multiple comparison test (GraphPad Software Inc., San Diego, CA, USA). A p-value of less than 0.05 was considered statistically significant.3 Results
3.1 Effect of the anti-oxidant and AuNP treatment on wound healing
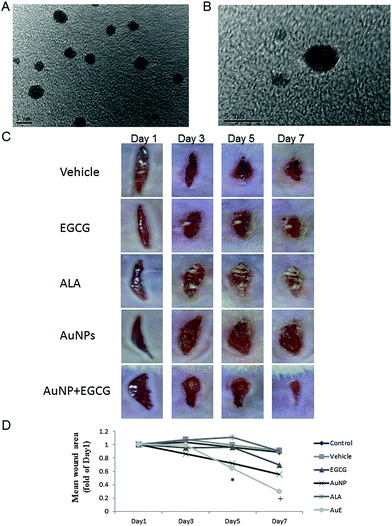
We tested the size and stability of the AuNPs in the culture medium. The diameter of the utilized AuNPs was found to be 3–5 nm by TEM analysis (Fig. 1A and B). For the investigation of the wound healing process after the gas-injection treatment in vivo, two linear wounds with full thickness were cut on the dorsum of the vehicle (control), EGCG, ALA, AuNP, and AuE (AuNP + EGCG) treated wild type (Fig. 1C) and diabetic (Fig. 2A) mice . Gas-injection of the AuE liquid significantly increased the rate of wound closure in wild type (Fig. 1D) and diabetic (Fig. 2B) mice. The wound area significantly decreased on day 7 in wild type mice and decreased from day 3 to day 7 in diabetic mice. The healing rate was also higher than in the vehicle group at 7 days after the gas-injection treatment. Direct topical AuE liquid treatment without gas-injection (as another control group) did not significantly accelerate the wound healing (data not shown). No significant adverse effects of the gas-injection treatment were noted on the general health of the mice.3.2 The effect on angiogenesis and Cu/Zn superoxide dismutase (SOD-1)
On the seventh day of the gas-injection treatment after injury, the vascular endothelial cell growth factor (VEGF) expression significantly increased in the diabetic mice (Fig. 3A). However, the VEGF protein did not significantly increase or decrease from day 3 to day 5. On the seventh day of treatment, all treatment groups showed an increase of the SOD-1 protein expression (data not show). In addition, the AuE gas-injection treatment significantly induced SOD-1 protein expression from day 3 to day 7 (Fig. 3B). These data indicate that gas-injection of the AuE formula treatment not only increases the VEGF expression around the wound area but also induces superoxide scavenging after injury in diabetic mice.3.3 Epidermal growth factor receptor (EGFR) and collagen protein expression in the diabetic wound area
Many factors result in reoccurring and continuous diabetic ulcers, and abnormal growth factor expression as well as angiogenesis is often considered to be important factors in diabetic ulcers. EGFR signaling is important in epidermal cell proliferation and cutaneous wound healing processes. Gas-injection of AuNPs and EGCG significantly increased the EGFR expression on day 5 and decreased on day 7 after cutaneous injury in diabetic mice (Fig. 4A), which may due to the final stage of wound closure in the AuE treated group. On the other hand, gas-injection of AuE did not induce collagen I & III protein expression on the first 3 days, but significantly increased on day 5 and day 7 around the diabetic wound area (Fig. 4B).3.4 Immunohistochemical staining for collagen and HA
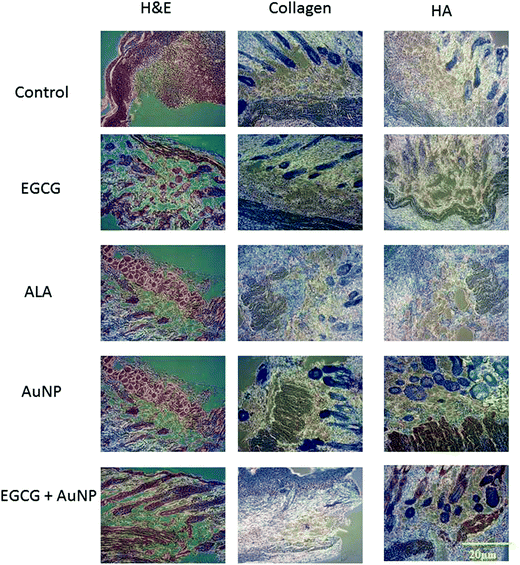
We collected the treated diabetic skin tissues for collagen and hyaluronic acid (HA) protein expression analysis by immunohistochemical staining. In the AuE group, the largest number of positives on collagen I & III was observed on day 7 after the cutaneous injury. Compared to other groups, the expression of HA in the AuE gas-injection group was significantly higher after 7 days in wild type (Fig. 5) and diabetic (Fig. 6) mice. These results indicate that gas-injection of AuE not only increases the collagen protein expression during the cutaneous wound healing process but also induces the HA expression around the wounded area.4. Discussion
High levels of blood glucose have been shown to be associated with many types of diabetic complications, because high blood glucose levels produces free radicals as well as creates conditions which favor oxidation.20 Oxidative stress is imperative for its morbidity towards diabetic complications, where abnormal metabolic milieu, as a result of hyperglycemia, leads to the onset of several complications. Therefore, materials that can eliminate peroxides could potentially control diabetic complications. These nanoscale constructs provide a range of multiple, fundamentally new properties, which can be exploited in ways that can improve our ability to detect, treat and monitor disease states.21 Further, the unique interactions between these nanoscale materials and comparably sized physiological structures, proteins, organelles and DNA,22,23 provides nanotechnology for potential biomedical applications. In this study, the results support the potential application of the gas-injection machine and the AuE formula in treating diabetic ulcers.Multiple factors cause recurrent, persistent, and refractory diabetic ulcers.24 Among them, impaired angiogenesis and growth factors have been considered to be important.25 Our previous studies have indicated that the local application of AuNPs combined with EGCG and ALA in diabetic ulcers promotes fibroblast proliferation and migration. This mixed formula has shown positive efficacy in the treatment of normal cutaneous wounds and diabetic ulcers.26,27 However, there are also some problems associated with the use of the EGCG mixed formula. The absorption ability and bioavailability of EGCG in diabetic ulcers is always a challenge.28,29 Thus, we wanted to introduce an appropriate absorption technique to retain the biological activity of AuE and maintain the continuous contact of AuE with the diabetic wound surfaces. Topical application of nucleic acids offers many potential therapeutic advantages for the suppression of genes in the skin, and potentially for systemic gene delivery.6,30,31 However, the epidermal barrier typically precludes the entry of gene-suppressing therapies unless the barrier is disrupted. AuNPs have been used in drug delivery and gene transfection for many years.32 Many studies have shown that using nanoparticles containing gold for drug delivery is a safe and non-toxic method that does not inactivate growth factors in the wound area.33 As a result of the concerns regarding the absorption rate and bioavailability of this formula, we tried a new method for the delivery of green tea extract directly into the wound. In this study, we used the GNT GoldMed™ Liquid Drug Delivery System to delivery AuE nanoparticles. It uses low gas pressure to deliver well-dispersed nanoparticle mixed liquid compounds, which allows for a large non-contact area with the wound surface. The pressure is essential for the potential injection processing of these gold nanoparticles. The anti-oxidants of the AuE were gas-injected into the cutaneous wounds and induced VEGF and SOD-1 expression after an initial burst during the first 3 days. The antioxidant effect could be extended up to 7 days and indicates that the nanoparticles can induce VEGF and EGFR expression, which is the fundamental factor to assure that angiogenesis promotes wound healing and contacts the granulation tissue. Furthermore, collagen protein and HA expressions also increased. These are both important components of the extracellular matrix and wound healing. These results suggest strongly that topical AuE treatment by gas-injection accelerates wound healing through the increase of the components in skin healing. The gas-injection of liquid AuE in specific concentrations could be simplified by the use of the GoldMed™ Liquid Drug Delivery System. Gas-injection of gold nanoparticles may be a better method than the topic application of ointments because this method may protect their biological activity in the deeper cutaneous areas.
EGCG supplementation greatly suppresses the diabetes-increased monocytes adhesion to endothelial cells, which is associated with reduced circulating levels of chemokines and reduced secretions of chemokines from db/db-EGCG mice. EGCG treatments reduce the nuclear translocation of NF-κB p65 in aortic vessels. EGCG may have a direct protective effect against vascular inflammation in diabetes.34 In db/db mice, EGCG improves the glucose tolerance and increases the glucose-stimulated insulin secretion.35 Collagen sponge incorporated with EGCG at low concentrations can enhance wound healing in diabetic mice by accelerating re-epithelialization and angiogenesis as well as improving the cellular reorganization of the granulation tissue by triggering the activity of myofibroblasts.36 In our histology data, the AuE gas-injection treatment showed anti-inflammatory effects in diabetic wounds. The healing rate in the AuE gas-injection group was the fastest among all groups. Pathologic slides also clearly showed that the AuE group displayed better collagen formation and tissue repair than the other groups. These results also support that gas-injection may deliver mixed compounds into deep cutaneous areas and maintain an effective concentration to promote wound healing without degradation of the active compounds by environmental enzymes. The biological antioxidant is capable of inhibiting the oxidative stress mediated diabetic progression. AuNPs have a profound control over antioxidant enzymes such as SOD in diabetic mice by inhibiting lipid peroxidation and ROS generation during hyperglycemia.37
In conclusion, this study presents a gold nanoparticles and a liquid drug delivery system to solve the problem of the low bioavailability of green tea extract. It enhances the ability of AuNPs to promote the formation of collagen and hyaluronic acid in the deep layers of the skin. Our study provides a new and more effective method for the future clinical delivery of other growth factors or antioxidants as topical treatments for diabetic ulcers.
Notes and references
- K. Knop, R. Hoogenboom, D. Fischer and U. S. Schubert, Angew. Chem., Int. Ed., 2010, 49, 6288–6308 CrossRef CAS PubMed.
- R. A. Petros and J. M. DeSimone, Nat. Rev. Drug Discovery, 2010, 9, 615–627 CrossRef CAS PubMed.
- E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-Sayed, Chem. Soc. Rev., 2012, 41, 2740–2779 RSC.
- S. Aggarwal, Nat. Biotechnol., 2009, 27, 987–993 CrossRef CAS PubMed.
- D. Kim, Y. Y. Jeong and S. Jon, ACS Nano, 2010, 4, 3689–3696 CrossRef CAS PubMed.
- D. A. Giljohann, D. S. Seferos, A. E. Prigodich, P. C. Patel and C. A. Mirkin, J. Am. Chem. Soc., 2009, 131, 2072–2073 CrossRef CAS PubMed.
- J. F. Hainfeld, F. A. Dilmanian, Z. Zhong, D. N. Slatkin, J. A. Kalef-Ezra and H. M. Smilowitz, Phys. Med. Biol., 2010, 55, 3045–3059 CrossRef CAS PubMed.
- Y. Jung, R. Reif, Y. Zeng and R. K. Wang, Nano Lett., 2011, 11, 2938–2943 CrossRef CAS PubMed.
- G. von Maltzahn, J. H. Park, A. Agrawal, N. K. Bandaru, S. K. Das, M. J. Sailor and S. N. Bhatia, Cancer Res., 2009, 69, 3892–3900 CrossRef CAS PubMed.
- A. J. Bard and L. R. Faulkner, Electrochemical methods: fundamentals and applications, Wiley, New York, 2001 Search PubMed.
- V. P. Torchilin, R. Rammohan, V. Weissig and T. S. Levchenko, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 8786–8791 CrossRef CAS PubMed.
- J. Park, T. Mattessich, S. M. Jay, A. Agawu, W. M. Saltzman and T. M. Fahmy, J. Controlled Release, 2011, 156, 109–115 CrossRef CAS PubMed.
- W. Jiang, B. Y. Kim, J. T. Rutka and W. C. Chan, Nat. Nanotechnol., 2008, 3, 145–150 CrossRef CAS PubMed.
- J. V. Higdon and B. Frei, Crit. Rev. Food Sci. Nutr., 2003, 43, 89–143 CrossRef CAS PubMed.
- J. M. Hodgson and K. D. Croft, Mol. Aspects Med., 2010, 31, 495–502 CrossRef CAS PubMed.
- Y. Hara, Green tea: health benefits and applications, Marcel Dekker, New York, 2001 Search PubMed.
- J. D. Lambert and C. S. Yang, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2003, 523–524, 201–208 CrossRef CAS.
- Z. Chen, Q. Y. Zhu, D. Tsang and Y. Huang, J. Agric. Food Chem., 2001, 49, 477–482 CrossRef CAS PubMed.
- C. Huo, S. B. Wan, W. H. Lam, L. Li, Z. Wang, K. R. Landis-Piwowar, D. Chen, Q. P. Dou and T. H. Chan, Inflammopharmacology, 2008, 16, 248–252 CrossRef CAS PubMed.
- T. Ma, J. Zhu, X. Chen, D. Zha, P. C. Singhal and G. Ding, Exp. Cell Res., 2013, 319, 779–789 CrossRef CAS PubMed.
- E. C. Dreaden, L. A. Austin, M. A. Mackey and M. A. El-Sayed, Ther. Delivery, 2012, 3, 457–478 CrossRef CAS.
- C. L. Zavaleta, B. R. Smith, I. Walton, W. Doering, G. Davis, B. Shojaei, M. J. Natan and S. S. Gambhir, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 13511–13516 CrossRef CAS PubMed.
- H. Wang, T. B. Huff, D. A. Zweifel, W. He, P. S. Low, A. Wei and J. X. Cheng, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 15752–15756 CrossRef CAS PubMed.
- M. Stucker, K. Harke, T. Rudolph and P. Altmeyer, Hautarzt, 2003, 54, 750–755 CrossRef CAS PubMed.
- E. Drela, K. Stankowska, A. Kulwas and D. Rosc, Adv. Clin. Exp. Med., 2012, 21, 249–254 Search PubMed.
- S. A. Chen, H. M. Chen, Y. D. Yao, C. F. Hung, C. S. Tu and Y. J. Liang, Eur. J. Pharm. Sci., 2012, 47, 875–883 CrossRef CAS PubMed.
- J. G. Leu, S. A. Chen, H. M. Chen, W. M. Wu, C. F. Hung, Y. D. Yao, C. S. Tu and Y. J. Liang, Nanomed.: Nanotechnol., Biol. Med., 2012, 8, 767–775 CrossRef CAS PubMed.
- J. Z. Xu, S. Y. Yeung, Q. Chang, Y. Huang and Z. Y. Chen, Br. J. Nutr., 2007, 91, 873–881 Search PubMed.
- N. T. Zaveri, Life Sci., 2006, 78, 2073–2080 CrossRef CAS PubMed.
- D. Zheng, D. A. Giljohann, D. L. Chen, M. D. Massich, X. Q. Wang, H. Iordanov, C. A. Mirkin and A. S. Paller, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 11975–11980 CrossRef CAS PubMed.
- N. L. Rosi, D. A. Giljohann, C. S. Thaxton, A. K. Lytton-Jean, M. S. Han and C. A. Mirkin, Science, 2006, 312, 1027–1030 CrossRef CAS PubMed.
- D. Pissuwan, T. Niidome and M. B. Cortie, J. Controlled Release, 2011, 149, 65–71 CrossRef CAS PubMed.
- P. Ghosh, G. Han, M. De, C. K. Kim and V. M. Rotello, Adv. Drug Delivery Rev., 2008, 60, 1307–1315 CrossRef CAS PubMed.
- P. V. Babu, H. Si and D. Liu, Mol. Nutr. Food Res., 2012, 56, 1424–1432 CAS.
- H. Ortsater, N. Grankvist, S. Wolfram, N. Kuehn and A. Sjoholm, Nutr. Metab., 2012, 9, 11 Search PubMed.
- H. Kim, T. Kawazoe, D. W. Han, K. Matsumara, S. Suzuki, S. Tsutsumi and S. H. Hyon, Wound Repair Regener., 2008, 16, 714–720 CrossRef PubMed.
- S. Barathmanikanth, K. Kalishwaralal, M. Sriram, S. R. Pandian, H. S. Youn, S. Eom and S. Gurunathan, J. Nanobiotechnol., 2010, 8, 16 CrossRef PubMed.
Footnote |
| † Yi-Huei Huang and Chao-Yi Chen contributed equally. |
| This journal is © The Royal Society of Chemistry 2014 |