 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High pressure differential scanning calorimetry of the hydrothermal salt solutions K2SO4–Na2SO4–H2O and K2HPO4–H2O
Joachim
Reimer
a and
Frédéric
Vogel
*ab
aPaul Scherrer Institute, Laboratory for Bioenergy and Catalysis, OVGA/104, CH-5232 Villigen PSI, Switzerland. E-mail: Frederic.vogel@psi.ch; Fax: +41 56 3102199; Tel: +41 56 3102135
bFachhochschule Nordwestschweiz, 5210 Windisch, Switzerland
First published on 3rd October 2013
Abstract
Aqueous salt solutions under hydrothermal conditions play an important role in geochemistry as well as in processes using hot compressed water as the process medium such as supercritical water oxidation (SCWO) and supercritical water gasification (SCWG). Isochoric high pressure differential scanning calorimetry (HP-DSC) was used as an accurate technique to investigate the phase behavior of such solutions under hydrothermal conditions. New data in the low concentration range (0.14–15.0 mass%) has been collected for the binary systems K2SO4–H2O, Na2SO4–H2O and K2HPO4–H2O. Furthermore we have discovered a liquid–liquid immiscibility in the ternary system K2SO4–Na2SO4–H2O, which has not been reported before.
Introduction
Aqueous salt solutions under hydrothermal conditions play an important role in geochemistry as well as in processes using hot compressed water as the process medium such as supercritical water oxidation (SCWO) and supercritical water gasification (SCWG). Salt solubility and phase behavior of the solutions are crucial e.g. for understanding ore formation1 and for the salt management in technical SCW processes.2–4At the Paul Scherrer Institute a hydrothermal gasification process was developed to produce methane from wet biomass.5 Inorganic compounds in the biomass feedstock can cause problems in the process by poisoning the methanation catalyst or lead to a blocking due to precipitation of solid salt at supercritical conditions. Therefore the inorganic salts have to be removed from the feed stream. Furthermore the separation of inorganic salts from the feed stream opens the possibility to recover nutrients such as phosphorus, potassium and ammonia, which could again be used as fertilizer in biomass production.
To optimize this salt separation process a detailed knowledge of the phase behavior of salt solutions is needed.
Historically, binary phase diagrams of aqueous salt solutions were divided into two major groups. The first group (type 1) has a continuous solubility line from the triple point of pure water to the triple point of the pure salt. Salts with type 1 behavior include e.g. halides and nitrates. The second group (type 2) is characterized by a solubility line that intersects the critical line in a lower critical endpoint p and a higher critical endpoint q. Between those invariant points the fluid is in equilibrium with the solid salt phase. Typical type 2 salts are sulfates, carbonates, silicates and oxides. Often phase diagrams of both types are complicated by liquid–liquid immiscibilities. Valyashko derived a set of phase diagrams for all possible cases.6
Aqueous Na2SO4 and K2SO4 solutions are known to have a phase diagram of type 2d′ (ref. 7) which possesses a metastable liquid–liquid immiscibility. K2HPO4–H2O is known for its type 1d behavior exhibiting a stable liquid–liquid immiscibility below the liquid 1-gas critical endpoint R.7 The schematic T–x projections of the two phase diagrams of interest (type 1d, 2d′) are shown in Fig. 1.
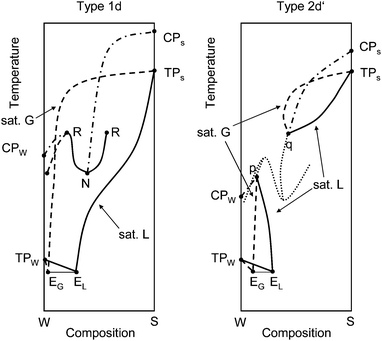 | ||
| Fig. 1 T–x projections of type 1d and 2d′ schematic phase diagrams. Bold lines – liquid composition at the three phase equilibrium (G–L–S and G–L1–L2); dashed lines – gas phase composition at the three phase equilibrium (G–L–S and G–L1–L2); dash-dotted lines – critical curves; dotted lines – metastable liquid–liquid immiscibility. EG – composition of gas phase at eutectic equilibrium (G–L–SW–SS); EL – composition of liquid phase at eutectic equilibrium (G–L–SW–SS); TPW, TPS – triple point of pure water and pure salt, respectively; CPW, CPS – critical points of pure water and pure salt, respectively; N – lower critical end-point (L1 = L2–G); R – upper critical end-point (L1 = G–L2); p, q – critical end-points (G = L–SS). Adapted from Valyashko.6 | ||
As in naturally occurring salt solutions typically more than one salt is dissolved, higher order mixtures are also of interest. In the phase diagrams of such solutions the properties of one binary mixture transform into the properties of the second binary mixture, and this leads to either continuous critical lines or invariant critical endpoints.6 Valyashko also developed a system to classify these phase diagrams.6 The ternary system K2SO4–Na2SO4–H2O was studied up to 200 °C by Freyer and Voigt8 who reported the formation of the double salt Glaserite of the formula NaK3(SO4)2 in certain concentration ranges, also with varying Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K ratios.
K ratios.
In earlier investigations from our group9–11 the continuous salt separation from hydrothermal salt solutions was investigated. Binary type 1 salt solutions showed high separation efficiencies, whereas type 2 solutions showed low separation efficiencies and caused deposition of solid salt in the apparatus. Interestingly, a ternary solution of the two type 2 salts Na2SO4 and K2SO4 could also be recovered efficiently. The salt deposition in the salt separation unit was studied with neutron radiography on the macro-scale.12–14 The salt precipitation and separation in connection with SCWG and SCWO processes was also studied in other groups using different approaches e.g. precipitation on a hot finger, special reactor designs (e.g. MODAR reactor, transpiring wall reactor).2,3,15–21 The results of these studies are often affected by the design of the used setup and restricted to the range of the parameters (p, T, x) varied.
For a better understanding of the phase behavior of salt solutions we used differential scanning calorimetry as a thermal analysis tool to investigate the phase transitions in such solutions. Thermal analysis is a sensitive and accurate tool to investigate phase transitions and phase behavior.22,23 Differential Scanning Calorimetry (DSC) is very sensitive towards phase transitions accompanied by changes in the heat capacity of the sample. The DSC measures the heat flow into or out of the sample versus a reference. Changes in the heat capacity of the sample due to phase transitions lead to step or peak shaped signals in the heat flow curves. The measured heat flow is proportional to the sample mass, the heating/cooling rate and the specific heat capacity (cV) of the sample (eqn (1)) and in addition to that affected by an instrumental baseline.
| Φmeasured = Φsample + Φbaseline = cvmβ + Φbaseline | (1) |
As a result of this relation the signal intensity increases with high scanning rates, but often smaller thermal effects vanish at such high rates due to intense broad signals of stronger effects. This led to problems in the interpretation of the heat flow curves in earlier investigations in our group24 where a very high scanning rate of 10 K min−1 had been chosen.
To test the method for accuracy we first measured salt solutions of Na2SO4, which have been studied extensively before.25–35 For K2SO4 and K2HPO4 much less data is available in the literature. Subsequently, measurements above 200 °C on the ternary system Na2SO4–K2SO4–H2O were performed.
Experimental section
All experiments were conducted on a Sensys DSC manufactured by SETARAM, France. The calorimeter is equipped with a Calvet-style sensor, which allows high precision measurements, since the sensor covers almost the whole sample leading to a higher amount of the heat flow being detected, compared to plate type DSC where the sensors only capture the heat flow through the bottom plate of the sample container. The temperature and energy calibration of the instrument were verified with samples of indium, tin, lead and zinc, to cover the whole temperature range of the investigations. All metals had a purity of >99.999%, except indium which had a purity of >99.995%, and were supplied by the manufacturer of the instrument.The salt solutions were prepared gravimetrically with an accuracy of ±0.1 mg and then weighed into the Incoloy crucibles with the same accuracy. Deionized water and salts with a purity >99.5% were used for the sample preparation. The crucibles are rated up to 50 MPa at 600 °C. The inner volume of the crucibles was determined to be 128.63 ± 0.54 μL (95% confidence interval) by fully filling them with water at 25 °C and weighing.
The crucibles were filled with 38.6 ± 0.1 mg of the liquid sample, and therefore the average density was 300 ± 1 kg m−3. An empty crucible was used as reference.
During the measurement the sensor was flushed with 20 mL min−1 of argon. The heating rate was 10 K min−1 for the heat-up phase. After an isothermal step of 1 h the measurement started with a heating/cooling rate of 0.1 K min−1.
The experimental data was treated with the Calisto Software (AKTS, Switzerland). Typically a smoothing of 25–50 points was necessary. Multiple measurements were performed to determine the uncertainty of the measurement.
The differences in the isochoric heat capacity (ΔcV) of the samples before and after a phase transition were determined using the glass transition tool of the Calisto software. This tool constructs tangents to the heat flow curves before and after a step-shaped transition and then calculates the ΔcV from the intersection points of those tangents with a tangent on the inflection point of the curve using the following relation (eqn (2)):
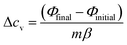 | (2) |
Results and discussion
Na2SO4–H2O
All the heat flow curves of the system Na2SO4–H2O show two signals. Upon heating the first signal is an endothermic peak. This signal is attributed to the appearance of a solid phase (G–L → G–L–S). Comparison of the heat flow curves with those obtained from experiments in the cooling mode showed that the precipitation of the solid salt is kinetically hindered when the measurement is performed in the heating mode and that substantial superheating occurs. Hence, the samples were remeasured in the cooling mode, showing much lower transition temperatures and step-like signals (Fig. 2). The second signal is an exothermic step and it is believed to be the disappearance of the gas phase or the liquid phase, leading to a one phase fluid (F) in equilibrium with a solid salt (G–L–S → F–S). The negative value of the difference in the isochoric heat capacity (ΔcV) underlines this assumption. The temperature for this transition at an average density of 300 kg m−3 and an initial salt concentration of 1.39 mass% was 375.2 ± 0.1 °C which is in accordance with the data of Valyashko et al.,35 who obtained in a similar experiment a transition temperature of 374.97 ± 0.17 °C for a 1 mass% solution at an average density of 309.9 kg m−3. The expected λ-shape of the second signal is less pronounced in the heating mode. In the cooling mode it is more pronounced.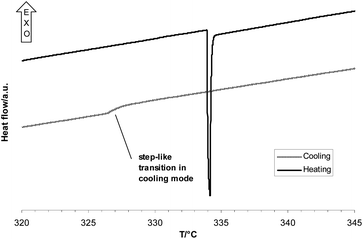 | ||
| Fig. 2 Comparison of the heat flow signals in heating and cooling mode at 0.1 K min−1, 6.69 mass% Na2SO4, average density 300 kg m−3. | ||
From the transition temperatures a part of the phase diagram can be constructed by correcting the concentration of the liquid phase with the method proposed by Valyashko et al.35 using the density data from Khaibullin and Novikov.29 This correction is needed because water evaporates from the starting solution during heating and therefore the salt concentration in the liquid phase increases. Concentrations of the salt in the vapor phase are normally very small compared to the concentration in the liquid phase and were thus neglected here.
The thermal expansion of the crucible was calculated from the material constants. The expansion of the crucibles due to pressure changes were taken into account. Furthermore we relied on the vapor density data available in the literature and did not use any data on the vapor density from databases or calculations. The uncertainties for the liquid concentration were estimated by calculation of the liquid concentration for extreme values of the liquid and vapor density, as proposed by Valyashko et al.35
Our corrected measurements are presented in Table 1. A comparison with literature data is shown in Fig. 3. The data obtained from the DSC measurements is in excellent accordance with the literature data. The metastable liquid–liquid immiscibility has not been observed. This is in accordance with Valyashko et al.35 suggesting the immiscibility to be suppressed by the appearance of the solid phase.
| Initial concentration/mass% | T/°C (G–L–S) | Liquid solution | Vapor solution | |
|---|---|---|---|---|
| x/mass% | ρ/kg m−3 | ρ/kg m−3 | ||
| a Obtained from interpolation of data from Khaibullin and Novikov.29 | ||||
| 0.140 | 372.4 ± 0.1 | 0.260 ± 0.005 | 435 ± 2a | 214 ± 2a |
| 0.696 | 360.7 ± 0.2 | 1.02 ± 0.01 | 540 ± 2a | 148 ± 2a |
| 1.39 | 352.5 ± 0.2 | 1.86 ± 0.02 | 594 ± 2a | 120 ± 2a |
| 2.75 | 342.2 ± 0.1 | 3.47 ± 0.02 | 652 ± 2a | 95.7 ± 2a |
| 6.69 | 326.6 ± 0.3 | 7.96 ± 0.05 | 755 ± 2a | 70.6 ± 2a |
| 15.0 | 305.7 ± 0.1 | 17.1 ± 0.1 | 884 ± 2a | 50.5 ± 2a |
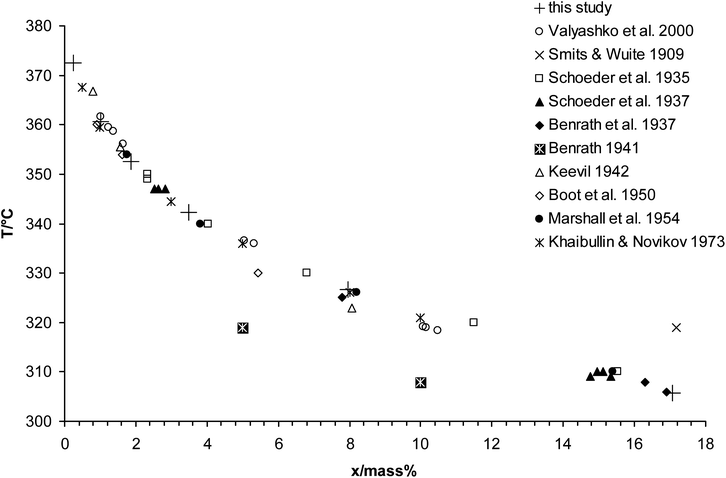 | ||
| Fig. 3 Temperature–composition plot for the liquid at the three-phase equilibrium (G–L–S) of the system Na2SO4 + H2O. | ||
K2SO4–H2O
K2SO4 is a type 2d′ salt like Na2SO4 and therefore the DSC experiments showed quite similar results. Interestingly the transition temperatures for the appearance of the solid phase are much higher compared to the sodium salt. The liquid concentration was corrected with the method mentioned before. Liquid density data was extrapolated from the data of Puchkov et al.36 Because of the lack of data for the vapor density, we extrapolated the vapor pressures for the saturated solutions from the data of Ravich et al.37 and used the corresponding vapor densities for pure steam obtained from the NIST database.38 The extrapolation of the liquid densities and the vapor pressures is subject to some uncertainty which we assume to be of the same magnitude as the uncertainty for the density measurements for the Na2SO4 system. Therefore we assumed an uncertainty for the densities of ±2 kg m−3. The uncertainty in the liquid concentration was estimated as mentioned before. The measured and calculated values are shown in Table 2.| Initial concentration/mass% | T/°C (G–L–S) | Liquid solution | Vapor solution | |
|---|---|---|---|---|
| x/mass% | ρ/kg m−3 | ρ/kg m−3 | ||
| a Obtained from extrapolation of data from Puchkov et al.36 b Obtained from extrapolation of the vapor pressures from Ravich et al.37 and calculation by the NIST Chemistry Webbook.38 | ||||
| 0.864 | 371.7 ± 0.2 | 1.69 ± 0.03 | 565 ± 2a | 196 ± 2b |
| 1.72 | 367.7 ± 0.1 | 2.90 ± 0.04 | 586 ± 2a | 173 ± 2b |
| 3.40 | 362.3 ± 0.6 | 5.19 ± 0.05 | 615 ± 2a | 148 ± 2b |
| 5.04 | 358.9 ± 0.1 | 7.34 ± 0.07 | 639 ± 2a | 137 ± 2b |
| 8.19 | 353.5 ± 0.1 | 11.4 ± 0.1 | 691 ± 2a | 121 ± 2b |
Our results are in good accordance with the few data sets available in the literature (Fig. 4), especially with the data of Ravich and Borovaya.39 Further measurements of densities are required to improve the correction of the measured DSC data. The DSC measurements also showed substantial superheating in this system, therefore all data was measured in the cooling mode.
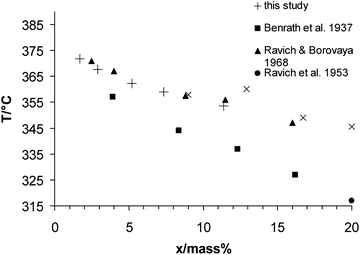 | ||
| Fig. 4 Temperature–composition plot for the liquid at the three-phase equilibrium (G–L–S) of the system K2SO4 + H2O. | ||
K2HPO4
In the heating mode the heat flow curves showed an endothermic step-like transition with a positive ΔcV value followed by a second exothermic step with a negative ΔcV value (Fig. 6). The first transition is assigned to the appearance of a second liquid phase, leading to a liquid 1 – liquid 2 – gas equilibrium. The second transition is assigned to the critical endpoint R, where the dilute liquid phase and the gas phase become equal. Supersaturation was also observed for the first transition, but to a much lower extent compared to the type 2 salts. For a solution with the concentration of 1.72 mass% the signal appeared at 372.3 ± 0.9 °C in the heating mode and at 371.4 ± 0.1 °C in the cooling mode. Hence, all the temperatures given in Table 3 were measured in the cooling mode.| Initial concentration/mass% | T/°C (G–L1–L2) |
|---|---|
| 0.434 | 376.7 ± 0.1 |
| 0.865 | 374.8 ± 0.1 |
| 1.72 | 371.4 ± 0.1 |
| 3.96 | 366.6 ± 0.1 |
| 6.46 | 364.2 ± 0.2 |
As in the measurements with the sulfate solutions the λ-shape of the second transition was less distinct in the heating mode compared to the cooling mode.
No phase density data is available in the literature for this system and therefore the correction for the liquid phase concentration could not be applied. Comparing our measurements to those of Marshall et al.,40,41 which were also taken at isochoric conditions, but probably at higher densities, we see a consistent trend of the data points (Fig. 5). This is especially the case if we assume that all data points in this study have to be corrected to higher concentrations.
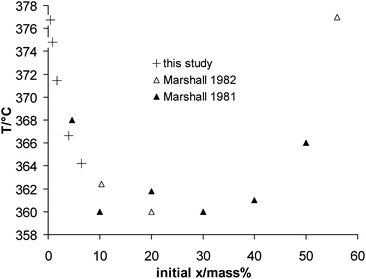 | ||
| Fig. 5 Temperature–initial composition plot for the liquid at the three-phase equilibrium (G–L1–L2) of the system K2HPO4 + H2O. | ||
K2SO4–Na2SO4–H2O
Interestingly, in our investigations of the ternary system K2SO4–Na2SO4–H2O we found heat flow curves resembling those of the measurements with the K2HPO4–H2O system. The first signal upon heating is an endothermic step with positive ΔcV value and the second signal is an exothermic step with negative ΔcV (Fig. 6). Therefore we assume that in mixed solutions of K2SO4 and Na2SO4 a liquid–liquid immiscibility occurs. The λ-shape of the second transitions was not clearly visible in the heating mode. As in the whole concentration range of our investigations (Table 4) we did not see any precipitation of a solid phase but only the liquid–liquid immiscibility, we conclude that the ternary solutions of potassium and sodium sulfate behave like a binary type 1d solution.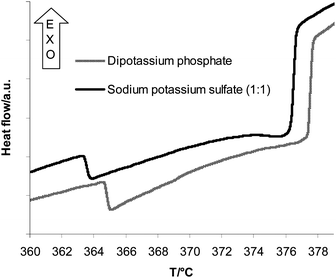 | ||
Fig. 6 Comparison of the heat flow curves of a K2HPO4 (6.46 mass%) solution and a Na–K SO4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution. 1) solution. | ||
| Initial concentration (Na2SO4)/mass% | Initial concentration (K2SO4)/mass% | Molar ratio K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na Na |
T/°C (G–L1–L2) | T/°C (G–L) |
|---|---|---|---|---|
| 0.699 | 1.71 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
367.79 ± 0.07 | 376.93 ± 0.03 |
| 1.40 | 0.857 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
363.20 ± 0.06 | 376.25 ± 0.01 |
| 1.39 | 1.70 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
363.54 ± 0.07 | 376.52 ± 0.02 |
| 0.688 | 3.38 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
367.09 ± 0.01 | 376.99 ± 0.01 |
The immiscibility may arise through the metastable states which are reported for the binary solutions of sodium and potassium sulfate. A similar effect has been reported for the system K2SO4–Li2SO4–H2O.42–44 This liquid–liquid immiscibility was observed at all molar ratios K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na from 1
Na from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 4
2 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Conclusions
Isochoric high pressure differential scanning calorimetry proved to be a relatively simple and fast method to investigate the phase behavior of aqueous salt solutions. It has the drawback that liquid and vapor densities have to be known to correct the data for the liquid concentration. Different phase transitions, such as the precipitation of a solid salt or the appearance of a second liquid phase, can easily be distinguished in the heating mode. We acquired new data in the low concentration range and reported the first calorimetric measurements on liquid–liquid immiscibilities in hydrothermal salt solutions. Furthermore we were able to find such phenomena also in the ternary K2SO4–Na2SO4–H2O system, which exhibits a pseudo type 1d behavior, even though it consists of two type 2 salts. Such changes in the phase behavior of mixed salt solutions offer an opportunity for optimizing the salt separation process for SCWO and SCWG applications, as type 1 salt solutions can be separated and recovered efficiently. Hence, the addition of additional salts to a feed solution might change the phase behavior e.g. of a type 2 solution in such a way that no solid salts precipitate and that the separation can be conducted successfully. Nevertheless more studies on higher order mixtures are necessary to explore the possibilities and limits of this proposed technique of enhancing salt separation in SCW processes. The lack of density data for gas and liquid phases at hydrothermal conditions is currently a limitation, as this data is needed to fully interpret the isochoric DSC measurements.Abbreviations
| G | Gas/vapor phase |
| L | Liquid phase |
| S | Solid phase |
| F | Supercritical fluid |
| c V | Isochoric specific heat capacity |
| Φ | Heat flow |
| β | Heating rate |
| m | Sample mass |
Acknowledgements
The authors are very grateful to S. Pin, T. Huthwelker, T. Driesner (ETH Zurich) and A. Wokaun for fruitful discussions. This work was financially supported by the Swiss National Science Foundation (SNF) under SNF grant #200021_132320.Notes and references
- D. Coumou, T. Driesner and C. A. Heinrich, Science, 2008, 321(5897), 1825–1828 CrossRef CAS PubMed.
- P. A. Marrone, M. Hodes, K. A. Smith and J. W. Tester, J. Supercrit. Fluids, 2004, 29(3), 289–312 CrossRef CAS.
- M. Hodes, P. A. Marrone, G. T. Hong, K. A. Smith and J. W. Tester, J. Supercrit. Fluids, 2004, 29(3), 265–288 CrossRef CAS.
- A. A. Peterson, F. Vogel, R. P. Lachance, M. Froling, M. J. Antal, Jr and J. W. Tester, Energy Environ. Sci., 2008, 1(1), 32–65 CAS.
- F. Vogel, in Handbook of Green Chemistry Volume 2 Heterogeneous Catalysis, ed. P. Anastas and R. Crabtree, Wiley-VCH, Weinheim, 2009, vol. 2, ch. 12, pp. 281–324 Search PubMed.
- V. M. Valyashko, in Hydrothermal Experimental Data, John Wiley & Sons, Ltd, 2008, pp. 1–133 Search PubMed.
- V. Valyashko, in Aqueous Systems at Elevated Temperatures and Pressures, ed. D. Palmer, R. Fernández-Prini and A. H. Harvey, Academic Press, London, 2004, pp. 597–641 Search PubMed.
- D. Freyer and W. Voigt, Geochim. Cosmochim. Acta, 2004, 68(2), 307–318 CrossRef CAS.
- M. Schubert, J. Aubert, J. B. Müller and F. Vogel, J. Supercrit. Fluids, 2012, 61, 44–54 CrossRef CAS PubMed.
- M. Schubert, J. W. Regler and F. Vogel, J. Supercrit. Fluids, 2010, 52(1), 99–112 CrossRef CAS PubMed.
- M. Schubert, J. W. Regler and F. Vogel, J. Supercrit. Fluids, 2010, 52(1), 113–124 CrossRef CAS PubMed.
- A. A. Peterson, P. Vontobel, F. Vogel and J. W. Tester, J. Supercrit. Fluids, 2008, 43(3), 490–499 CrossRef CAS PubMed.
- A. A. Peterson, P. Vontobel, F. Vogel and J. W. Tester, J. Supercrit. Fluids, 2009, 49(1), 71–78 CrossRef CAS PubMed.
- A. A. Peterson, J. W. Tester and F. Vogel, J. Supercrit. Fluids, 2010, 54(2), 250–257 CrossRef CAS PubMed.
- I. Leusbrock, S. J. Metz, G. Rexwinkel and G. F. Versteeg, J. Supercrit. Fluids, 2010, 53(1–3), 17–24 CrossRef CAS PubMed.
- I. Leusbrock, S. J. Metz, G. Rexwinkel and G. F. Versteeg, J. Supercrit. Fluids, 2010, 54(1), 1–8 CrossRef CAS PubMed.
- G. Akgul and A. Kruse, J. Supercrit. Fluids, 2012, 66, 207–214 CrossRef PubMed.
- A. Kruse, D. Forchheim, M. Gloede, F. Ottinger and J. Zimmermann, J. Supercrit. Fluids, 2010, 53(1–3), 64–71 CrossRef CAS PubMed.
- F. J. Armellini and J. W. Tester, Fluid Phase Equilib., 1993, 84, 123–142 CrossRef CAS.
- F. J. Armellini, J. W. Tester and G. T. Hong, J. Supercrit. Fluids, 1994, 7(3), 147–158 CrossRef CAS.
- P. Whiting, U.S. Pat., 5667698 A, 1997.
- I. Abdulagatov, V. Dvoryanchikov, B. Mursalov and A. Kamalov, Fluid Phase Equilib., 1998, 143, 525–535 CrossRef.
- K. G. Kravchuk and K. Todheide, Z. Phys. Chem., 1996, 193, 139–150 CrossRef CAS.
- A. A. Rouff, S. Rabe and F. Vogel, J. Supercrit. Fluids, 2011, 57(3), 207–212 CrossRef CAS PubMed.
- A. Benrath, Z. Anorg. Allg. Chem., 1941, 247, 147–160 CrossRef CAS.
- A. Benrath, F. Gjedebo, B. Schiffers and H. Wunderlich, Z. Anorg. Allg. Chem., 1937, 231, 285–297 CrossRef CAS.
- H. Booth and R. Bidwell, J. Am. Chem. Soc., 1950, 72, 2567–2575 CrossRef CAS.
- N. Keevil, J. Am. Chem. Soc., 1942, 64, 841–850 CrossRef CAS.
- I. K. Khaibullin and B. Novikov, High Temp., 1973, 11, 276–282 Search PubMed.
- W. L. Marshall, H. Wright and C. Secoy, J. Chem. Educ., 1954, 31, 34–36 CrossRef CAS.
- W. Schroeder, A. Berk and E. Partridge, J. Am. Chem. Soc., 1935, 57, 1783–1790 CrossRef.
- W. Schroeder, A. Berk and E. Partridge, J. Am. Chem. Soc., 1937, 59, 1790–1795 CrossRef CAS.
- A. Smits and J. Wuite, Proc. R. Soc. Amsterdam, 1909, 12, 244–257 Search PubMed.
- W. Tilden and W. Shenstone, Proc. R. Soc. London, Ser. A, 1883, 35, 345–346 CrossRef.
- V. M. Valyashko, I. M. Abdulagatov and J. M. H. Levelt Sengers, J. Chem. Eng. Data, 2000, 45(6), 1139–1149 CrossRef CAS.
- L. V. Puchkov, V. V. Kurochkina, and R. P. Matveeva, Deposit. VINITI USSR, 1976, n.3474–76 Dep., p. 20 Search PubMed.
- M. I. Ravich, F. Borovaya and V. Y. Ketkovich, Izv. Sekt. Fiz.-Khim. Anal., Inst. Obshch. Neorg. Khim., Akad. Nauk SSSR, 1953, 20, 225–239 Search PubMed.
- NIST Chemistry WebBook, NIST Standard Reference Database Number 69, ed. P. J. Linstrom and W. Mallard, National Institute of Standards and Technology, retrieved February 13, 2013 Search PubMed.
- M. I. Ravich and F. E. Borovaya, Zh. Neorg. Khim., 1968, 13, 1418–1425 CAS.
- W. L. Marshall, C. Hall and R. E. Mesmer, J. Inorg. Nucl. Chem., 1981, 43, 722–728 CrossRef.
- W. L. Marshall, J. Chem. Eng. Data, 1982, 27(2), 175–180 CrossRef CAS.
- M. Ravich and V. Valyashko, Zh. Neorg. Khim., 1969, 14, 1650–1654 CAS.
- M. Urusova and V. Valyashko, Russ. J. Phys. Chem., 2012, 57(4), 579–591 CAS.
- V. M. Valyashko, Zh. Neorg. Khim., 1975, 20(4), 1129–1131 CAS.
| This journal is © The Royal Society of Chemistry 2013 |
