Selective continuous flow extractive denitrogenation of oil containing S- and N-heteroaromatics using metal-containing ionic liquids supported on monolithic silica with hierarchical porosity†
Abstract
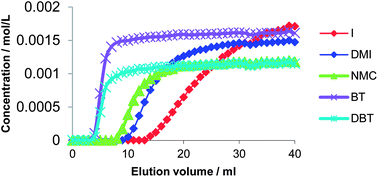
The removal of heteroaromatic nitrogen and sulfur impurities from a model oil through extraction with ionic liquids (ILs) containing metal salts was performed in view of the purification of fuel feeds. Chloride and bis(trifluoromethanesulfonyl)imide salts of Cu+, Cu2+ and Fe3+ were used. The systems based on ILs and metal compounds were applied both in batch-like liquid–liquid and continuous flow liquid–solid conditions. In a first phase, liquid–liquid biphasic extraction was used to choose the most adequate IL classes; the suitable systems were immobilized on a solid support to form metal-containing supported ionic liquid phases (SILPs). In a next phase, these SILPs were applied in breakthrough experiments. A selective extraction of N-compounds was achieved with metal-containing ionic liquids, in both liquid–liquid and liquid–solid conditions. The breakthrough experiments using Cu(NTf2)2− and FeCl4−-containing [BMIM][NTf2] SILPs immobilized on hierarchically structured silica monoliths resulted in an efficient separation of all the nitrogen compounds from the other impurities in the model oil.

 Please wait while we load your content...
Please wait while we load your content...