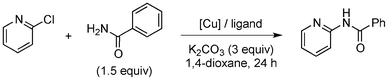
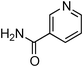
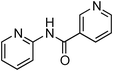
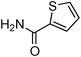
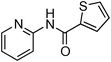
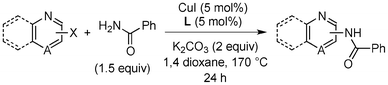
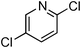
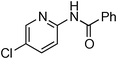
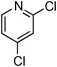
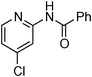
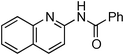
Copper-catalysed amidation of 2-chloro-pyridines†
Lionel Nicolasa,
Patrick Angibaudb,
Ian Stansfieldb,
Lieven Meerpoelc,
Sébastien Reymond*a and
Janine Cossy*a
aLaboratoire de Chimie Organique, ESPCI ParisTech, UMR CNRS 7084, 10, rue Vauquelin, 75231, Paris Cedex 05, France. E-mail: janine.cossy@espci.fr; sebastien.reymond@espci.fr; Fax: (+33)140794660; Tel: (+33)140794659
bJanssen Research & Development, a division of Janssen-Cilag, BP615- Chaussée du Vexin, 27106, Val de Reuil, France
cJanssen Research & Development, a division of Janssen Pharmaceutica N.V. Turnhoutsweg 30, 2340, Beerse, Belgium
First published on 19th July 2013
Abstract
The simple and inexpensive N,N-dimethylcyclohexane-1,2-diamine/CuI catalytic system provides a versatile, easy and efficient access to an array of N-(2-pyridin-2-yl)-amides from 2-chloro-pyridine derivatives.
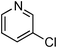
Amide formation is ubiquitous in organic chemistry as many biologically-relevant synthetic and natural products incorporate an amide moiety. Among amides, N-heteroaryl amides constitute one important class of pharmacophores used in medicinal chemistry and, recently, N-(2-pyridin-2-yl)-amide derivatives were reported to block sodium channels which are involved in neuronal regulation with potential applications in the treatment of pain, arrhythmia or epilepsy.1 Non-catalytic amidations of 2-amino heterocycles as well as metal-catalysed amidations of aryl halides are existing to access amide derivatives.2 However, despite considerable progresses in palladium- and copper-catalysed C–N bond formation,3–5 broadening the scope of electrophiles and nucleophiles that can be used in these reactions, only a few methods are able to achieve the amidation of aryl chlorides, and a few examples are reported for the amidation of 2-chloro-pyridine derivatives which are less reactive than their brominated counterparts.6 Herein, we would like to report a general method for the catalytic amidation of chloro-pyridine derivatives involving a cheap and simple catalytic system based on CuI and trans-N,N-dimethyl-cyclohexane-1,2-diamine.6b–c,f,7,8,9
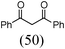
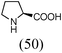
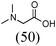
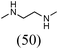
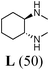
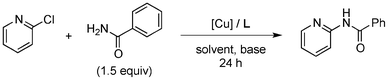
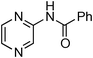
Initially, a catalytic amidation of 2-chloropyridine with benzamide was examined to tune up the reaction conditions (Table 1). Initial trials involving CuI (50 mol%) and 1,3-diphenylpropan-1,3-dione, proline or N,N-dimethylglycine as ligands (50 mol%) did not lead to any conversion of the starting materials (Table 1, entries 1–3). However, the use of N,N-dimethyl-ethylenediamine as a ligand (50 mol%) provided N-(pyridin-2-yl)benzamide in 48% yield (Table 1, entry 4), and the yield was increased to 82% when N,N-dimethylcyclohexane-1,2-diamine (50 mol%) was used (Table 1, entry 5).
Having identified N,N-dimethylcyclohexane-1,2-diamine (L) as the best ligand, the optimisation of the amidation of 2-chloropyridine with benzamide was achieved (Table 2). Other copper sources such as CuO, CuBr, Cu2O or Cu(OAc)2·H2O were evaluated (Table 2, entries 1–4), however they displayed lower catalytic activities than CuI (Table 1, entry 5). The use of K3PO4 as a base, instead of K2CO3, led to a slightly decreased yield (74%) whereas the use of Cs2CO3 lowered the yield to 36% (Table 2, entries 5–6). When DMF was used as the solvent, N-(pyridin-2-yl)benzamide was isolated in 48% yield, and when DME was utilised, the yield was similar to the one obtained with 1,4-dioxane (85%) (Table 2, entries 7–8). A decrease of the catalytic loading in both CuI and ligand L from 50 mol% to 10 mol% dramatically decreased the yield in the cross-coupling; N-(pyridin-2-yl)benzamide was isolated in 71% yield with 25 mol%, and in 28% yield with a 10 mol% catalytic loading (Table 2, entries 9–10). Gratifyingly, when the reaction was performed with 10 mol% CuI and 10 mol% L in 1,4-dioxane in a sealed tube at 170 °C, N-(pyridin-2-yl)benzamide was isolated in 81% yield (Table 2, entry 11) and, at this temperature, the amount of K2CO3 could be reduced to 2 equivalents. It was possible to decrease the catalytic charge to 5 mol% of CuI and 5 mol% of L as N-(pyridin-2-yl)benzamide was still obtained in good yield (69%) (Table 2, entry 12). With 2 mol% of CuI and 2 mol% of L, the yield was 60% however, after 60 h of reaction (Table 2, entry 13). We have to point out that in the absence of either CuI or L, no conversion of the starting material was observed under the reaction conditions (Table 2, entries 14–16).10
|
||||||
|---|---|---|---|---|---|---|
| Entry | [Cu] (mol%) | L (mol%) | Base (equiv.) | Solventa | T (°C) | Yieldb |
| a c = 1 M.b Isolated yield.c Reaction performed in a sealed tube.d After 60 h of reaction. | ||||||
| 1 | CuO (50) | (50) | K2CO3 (3) | 1,4-dioxane | 100 | 11% |
| 2 | CuBr (50) | (50) | K2CO3 (3) | 1,4-dioxane | 100 | 23% |
| 3 | Cu2O (50) | (50) | K2CO3 (3) | 1,4-dioxane | 100 | 38% |
| 4 | Cu(OAc)2·H2O (50) | (50) | K2CO3 (3) | 1,4-dioxane | 100 | 26% |
| 5 | CuI (50) | (50) | K3PO4 (3) | 1,4-dioxane | 100 | 74% |
| 6 | CuI (50) | (50) | Cs2CO3 (3) | 1,4-dioxane | 100 | 36% |
| 7 | CuI (50) | (50) | K2CO3 (3) | DMF | 150 | 38% |
| 8 | CuI (50) | (50) | K2CO3 (3) | DME | 85 | 85% |
| 9 | CuI (25) | (25) | K2CO3 (3) | 1,4-dioxane | 100 | 71% |
| 10 | CuI (10) | (10) | K2CO3 (3) | 1,4-dioxane | 100 | 28% |
| 11 | CuI (10) | (10) | K2CO3 (2) | 1,4-dioxane | 170c | 81% |
| 12 | CuI (5) | (5) | K2CO3 (2) | 1,4-dioxane | 170c | 69% |
| 13 | CuI (2) | (2) | K2CO3 (2) | 1,4-dioxane | 170c | 60%d |
| 14 | — | — | K2CO3 (2) | 1,4-dioxane | 170c | — |
| 15 | CuI (10) | — | K2CO3 (2) | 1,4-dioxane | 170c | — |
| 16 | — | (10) | K2CO3 (2) | 1,4-dioxane | 170c | — |
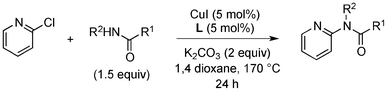
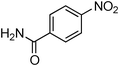
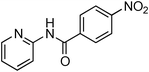
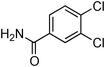
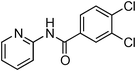
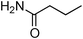
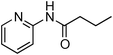
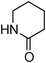
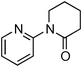
Having obtained optimized conditions for the cross-coupling of 2-chloropyridine with benzamide (5 mol% CuI and 5 mol% L, 2 equiv., K2CO3, 170 °C, 1,4-dioxane, 24 h), the reaction of 2-chloropyridine was evaluated with several aromatic, heteroaromatic and aliphatic amides, and the results are reported in Table 3. Aromatic amides such as 4-methoxybenzamide, 4-methylbenzamide or 4-nitrobenzamide provided the corresponding cross-coupling products in 72–57% yield (Table 3, entries 1–3), the electron-poor 4-nitrobenzamide leading to the lowest yield (57%) (Table 3, entry 3). When 3,4-dichlorobenzamide was engaged in the amidation of 2-chloropyridine, the cross-coupling was chemoselective and the expected amide was obtained in 69% yield (Table 3, entry 4). Heteroaromatic amides such as nicotinamide or thiophene-2-carboxamide are suitable in the cross-coupling with 2-chloropyridine as the corresponding amides were isolated with good yields of 83% and 75% respectively (Table 3, entries 5 and 6). Aliphatic amides such as a primary amide, butyramide, or a secondary amide, piperidin-2-one, provided the expected N-(pyridin-2-yl)amides in 96% and 78% yields respectively (Table 3, entries 7–8).
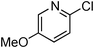
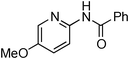
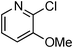
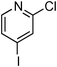
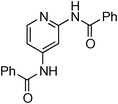
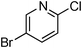
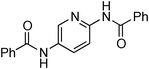
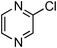
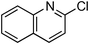
At this stage, the amidation of other pyridyl halides with benzamide was examined, under the optimized conditions (5 mol% CuI and 5 mol% L, 2 equiv. K2CO3, 170 °C, 1,4-dioxane, 24 h), and the results are reported in Table 4. When 2-chloro-5-methoxypyridine was used, N-(5-methoxypyridin-2-yl)benzamide was obtained in 92% yield (Table 4, entry 1), whereas with 2-chloro-3-methoxypyridine, no cross-coupling product was isolated and the starting material was recovered (Table 4, entry 2). The reaction of 2-chloro-5-chloropyridine was chemoselective and the expected mono-amide was obtained selectively, however in 45% isolated yield for 75% conversion of the starting dihalopyridine (Table 3, entry 3). When 2,4-dichloropyridine was used, the corresponding mono-amide was obtained chemoselectively and, in this case, the isolated yield was 32%, for 72% conversion of the starting 2,4-dichloro-pyridine (Table 3, entry 4). In contrast, the use of 2-chloro-4-iodopyridine led to the expected diamide in 32% yield and only traces of the mono-amide were observed (Table 3, entry 5). Concerning 2-chloro-4-bromopyridine, the diamide product was isolated in only 21% yield, and only traces of the mono-amide were observed (Table 3, entry 6). Worthy of note is the reaction of 3-chloropyridine which was unreactive under the reaction conditions (Table 3, entry 7). Finally, 2-chloropyrazine and 2-chloroquinoline successfully reacted with benzamide, and the corresponding amides were produced in 67% and 88% yield respectively (Table 3, entries 8–9).
Conclusions
In summary, we have described a straightforward method for the amidation of 2-chloropyridine derivatives with a cheap and convenient CuI/N,N-dimethylcyclohexane-1,2-diamine catalytic system, which constitutes an interesting alternative to both the reported Pd-centered methods and Cu-catalysed amidation of 2-bromo-pyridine derivatives. This C–N bond formation is general and can involve aromatic, heteroaromatic or aliphatic amides, and various pyridine derivatives such as 2-chloropyridines, as well as 2-chloropyrazine and 2-chloroquinoline.Acknowledgements
Janssen Research & Development, a Division of Janssen-Cilag, is gratefully acknowledged for financial support.References
- C. Ni, M. Park, B. Shao, L. Tafesse, J. Yao, M. Youngman and X. Zhou, WO 2012/085650 A1, 2012 Search PubMed.
- The stoichiometric amidation of 2-amino heterocycles is not always an obvious reaction because of their low nucleophilicity. For examples, see (a) A. R. Katritzky, H.-Y. He and K. Suzuki, J. Org. Chem., 2000, 65, 8210 CrossRef CAS PubMed; (b) A. R. Katritzky, B. El-Dien, M. El-Gendy, E. Todadze and A. A. A. Abdel-Fattah, J. Org. Chem., 2008, 73, 5442 CrossRef CAS PubMed; (c) K. Kim and K. Le, Synlett, 1999, 1957 CrossRef CAS.
- (a) A. Correa and C. Bolm, Topics in Organometallic Chemistry, Metal-catalyzed C(sp2)-N bond Formation, Springer, Berlin, Heidelberg, 2013 Search PubMed; (b) Y. Jiang and D. Ma, Topics in Organometallic Chemistry, Assembly of N-Containing Heterocycles via Pd- and Cu-Catalyzed C–N Bond Formation Reactions, Springer, Berlin, Heidelberg, 2013 Search PubMed; (c) I. P. Beletskaya and A. V. Cheprakov, Organometallics, 2012, 31, 7753 CrossRef CAS.
- For reviews on Pd-catalysed C-N bond formations, see (a) J. F. Hartwig, Nature, 2008, 455, 314–322 CrossRef CAS PubMed; (b) Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 2nd edn, 2004, vol. 1, ch. 13, p. 699 Search PubMed; (c) R. J. Lundgren and M. Stradiotto, Chem.–Eur. J., 2012, 18, 9758 CrossRef CAS PubMed; (d) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318 CrossRef CAS PubMed.
- For reviews on Cu-catalysed C-N bond formations, see (a) G. Evano, N. Blanchard and T. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef CAS PubMed; (b) I. P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337 CrossRef CAS; (c) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954 CrossRef CAS PubMed.
- For some examples, see (a) Q. Shen, S. Shekhar, J. P. Stambuli and J. F. Hartwig, Angew. Chem., Int. Ed., 2005, 44, 1371 CrossRef CAS PubMed; (b) F. Halley, Y. El-Ahmad, V. Certal, C. Venot, A. Dagallier, H. Strobel, K. Ritter and S. Ruf, US 2009/0082329 A1, 2009 Search PubMed; (c) N. R. Irlapati, G. K. Deshmukh, V. P. Karche, S. M. Jachak, N. Sinha, V. P. Palle and R. K. Kamboj, WO 2012/056478 A1, 2012 Search PubMed; (d) S. Guo, Y. Wang, C. Sun, J. Li, D. Zhou, Y. Wu and Y. Wu, Tetrahedron Lett., 2013, 54, 3233 CrossRef CAS; (e) H. Kakuta, X. Zheng, H. Oda, S. Harada, Y. Sugimoto, K. Sasaki and A. Tai, J. Med. Chem., 2008, 51, 2400 CrossRef CAS PubMed; (f) B. Wu, K. Kuhen, T. Ngoc Nguyen, D. Ellis, B. Anaclerio, X. He, K. Yang, D. Karanewsky, H. Yin, K. Wolff, K. Bieza, J. Caldwell and Y. He, Bioorg. Med. Chem. Lett., 2006, 16, 3430 CrossRef CAS PubMed; (g) G. B. W. L. Ligthart, H. Ohkawa, R. P. Sijbesma and E. W. Meijer, J. Org. Chem., 2006, 71, 375 CrossRef CAS PubMed; (h) D. Doller, G. Li, G. Ma and H. Zhou, US 2011/0098299 A1, 2011 Search PubMed; (i) P.-S. Wang, C.-K. Liang and M.-k. Leung, Tetrahedron, 2005, 61, 2931 CrossRef CAS.
- For examples of Cu-catalysed amidation of aryl halides, see (a) A. Klapars, J. C. Antilla, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 7727 CrossRef CAS PubMed; (b) K. R. Crawford and A. Padwa, Tetrahedron Lett., 2002, 43, 7365 CrossRef CAS; (c) S. K. Kang, D. H. Kim and J. N. Park, Synlett, 2002, 427 CrossRef CAS; (d) A. Klapars, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 7421 CrossRef CAS PubMed; (e) W. Deng, Y.-F. Wang, Y. Zou, L. Liu and Q.-X. Guo, Tetrahedron Lett., 2004, 45, 2311 CrossRef CAS; (f) E. R. Strieter, D. G. Blackmond and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 4120 CrossRef CAS PubMed; (g) W. Chen, J. Li, D. Fang, C. Feng and C. Zhang, Org. Lett., 2008, 10, 4565 CrossRef CAS PubMed; (h) E. R. Strieter, B. Bhayana and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 78 CrossRef CAS PubMed; (i) P. F. Larsson, A. Correa, M. Carril, P. O. Norrby and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5691 CrossRef CAS PubMed; (j) S. Jammi, S. Krishnamoorthy, P. Saha, D. S. Kundu, S. Sakthivel, M. A. Ali, R. Paul and T. Punniyamurthy, Synlett, 2009, 3323 CAS; (k) Y. Zijian and W. Xianwen, Chin. J. Chem., 2010, 28, 2260 CrossRef; (l) H. C. Ma, X. Z. Jiang, X. Ma and Z. Jiang, Synlett, 2008, 1335 CAS; (m) C. Wang, L. Liu, W. Wang, D.-S. Ma and H. Zhang, Molecules, 2010, 15, 1154 CrossRef CAS PubMed; (n) W. Mangang, Y. Hua, Y. Xinwen, W. Jun and S. Zhicai, Chin. J. Chem., 2012, 30, 2356 CrossRef; (o) H. Xu and C. Wolf, Chem. Commun., 2009, 1715 RSC.
- For selected examples involving the Cu-catalysed amidation of 2-bromo-pyridine, see (a) I. M. Bella, R. A. Bednarb, J. F. Fayb, S. N. Gallicchioa, J. H. Hochmanc, D. R. McMastersd, C. Miller-Steinc, E. L. Mooreb, S. D. Mosserb, N. T. Pudvahc, A. G. Quigleya, C. A. Salvatoreb, C. A. Stumpa, C. R. Thebergea, B. K. Wongc, C. B. Zartmana, X.-F. Zhanga, S. A. Kaneb, S. L. Grahama, J. P. Vaccaa and T. M. Williamsa, Bioorg. Med. Chem. Lett., 2006, 16, 6165 CrossRef PubMed; (b) J. Li, Y. Zhang and D. Ma, Tetrahedron Lett., 2012, 53, 3981 CrossRef CAS; (c) T. Hafner and D. Kunz, Synlett, 2007, 1403 CAS; (d) C.-C. Lee, P.-S. Wang, M. B. Viswanath and M.-k. Leung, Synthesis, 2008, 1359 CAS; (e) X. Lv and W. Bao, J. Org. Chem., 2007, 72, 3863 CrossRef CAS PubMed.
- For references concerning mechanistic features of Cu-catalysed C-N bond forming reactions, see ref. 3c, 6f, 6h and G. Lefèvre, G. Franc, A. Tlili, C. Adamo, M. Taillefer, I. Ciofini and A. Jutand, Organometallics, 2012, 31, 7694 CrossRef.
- The use of a large excess of primary amides such as benzamide was shown to convert 2-chloroquinolines and some halogenated quinolines, pyrazines and pyridines into the corresponding amino derivatives. With a large excess of acetamide (ca. 80 equiv.) it is possible to isolate the corresponding 2-acetamidoquinoline derivatives, however in modest yields. See (a) T. Watanabe, E. Kikuchi, W. Tamura, Y. Akita, M. Tsutsui and A. Ohta, Heterocycles, 1980, 14, 287 CrossRef CAS; (b) F. Kóródi, Synth. Commun., 1991, 21, 1841 CrossRef; (c) S. Inglis, R. Jones, D. Fritz, C. Stojkoski, G. Booker and S. Pyke, Org. Biomol. Chem., 2005, 3, 2543 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data and NMR spectra for amide compounds. See DOI: 10.1039/c3ra43368d |
| This journal is © The Royal Society of Chemistry 2013 |