 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rhenium(I) phenanthrolines bearing electron withdrawing CF3 substituents: synthesis, characterization and biological evaluation†
Carl
Redshaw
*a,
Scott
Watkins
b,
Simon M.
Humphrey
c,
Philip C.
Bulman Page
d,
Shane
Ashby
d,
Yimin
Chao
d,
Christopher J.
Herbert
d and
Anja
Mueller
*e
aDepartment of Chemistry, University of Hull, Cottingham Rd, Hull, UK HU6 7RX. E-mail: c.redshaw@hull.ac.uk
bCSIRO Materials Science and Engineering, Flexible Electronics Theme, Bayview Avenue, Clayton South, 3169, Victoria, Australia
cDepartment of Chemistry, The University of Texas at Austin, Welch Hall 2.204, 105 E. 24th St. A5300, Austin, TX 78712-1224, USA
dSchool of Chemistry, University of East Anglia, Norwich, UK NR4 7TJ
eSchool of Pharmacy, University of East Anglia, Norwich, UK NR4 7TJ. E-mail: anja.mueller@uea.ac.uk
First published on 4th October 2013
Abstract
Rhenium(I) tricarbonyl complexes bearing a 2,4,7,9-tetraphenylphenanthroline ligand, where the 2,9-phenyl groups have either meta- or para-CF3 groups, have been screened against a selection of cell lines. The meta derivative shows anticancer activity against HeLa and A549 cell lines, whereas the para derivative causes proliferation of HL-60 cells, whilst showing toxicity towards A549 cells.
One of the main goals in the field of metal-based anti-tumor drugs is to overcome the major limitations associated with cisplatin, namely drug resistance, poor selectivity and adverse side effects.1 Amongst the metals under consideration, rhenium has shown promising apoptosis and anti-tumor activity in a variety of oxidation states.2 Furthermore, rhenium complexes have become the logical choice for therapeutic applications, given the close similarities with the chemistry of technetium,3 and a number of complexes are now under clinical consideration.4 Whilst the most extensive chemistry reported for these group VII congeners involves the [MO]3+ core (metal in oxidation state +V), other studies have highlighted the potential of the organometallic fragment [fac-Re(CO)3]+.5 This fragment is readily accessible as the air stable [Re(CO)3(H2O)3]+ species, for which the aqua ligands are substitutionally labile. Furthermore, the small size of the core allows for tagging with bioactive moieties, whilst retaining bioactivity and specificity. Radio-imaging techniques do not permit visualization at the cellular level, however by replacing the radioisotope with a fluorophore, it is possible to use fluorescent microscopy to identify cellular targets and thereby correlate results with in vivo radio-imaging experiments. A number of conjugated chelating ligands have recently been bound to the [fac-Re(CO)3] core, including benzoimidazole, diimine and indole.6 To-date, results for the diimine systems have been restricted due to limited visible adsorption (generally emit only in the yellow/green region),7 this despite reports on rhenium(I) tricarbonyl diimines of the type [Re(CO)3(N–N)X] (N–N = diimine, X = halide) dating back to the 1970s.8 Given the relatively low sensitivity of the HOMO energy to changes to the diimine ligand, the emission energy of the rhenium(I) complexes is, broadly speaking, proportional to the reduction potential of the ligand. The colour of the emission can therefore be shifted by lowering the LUMO energy, i.e. by making the ligand reduction potential more positive – the so-called energy-gap law.9 One way in which this can be accomplished is to introduce electron withdrawing substituents at the ligand, thereby shifting the emission toward the red. Transitions to a ligand localized LUMO has recently been utilized in azadipyrromethane rhenium tricarbonyl complexes to bring about red emission.10 In the case of phenanthroline ligand systems, this can be achieved by the addition of phenyl groups, for example anionic complexes of the type [Re(CO)3(bathophenanthroline sulphate)(py-3-R)] (R = H, CH2OH; bathophenanthroline = 4,7-diphenyl-1,10-phenanthroline) exhibit red shifts of about 20 nm for excitation and 10 nm for emission.11 It has been noted in related biquinoline chemistry that the use of electron withdrawing substituents can also lead to red shifts.12 It was therefore expected that for bathophenanthroline-derived systems, phenyl groups possessing fluorine containing substituents such as CF3 groups would produce a red shift. The effect is more pronounced if the aryl group is not positioned meta to the Re–N bond. This red emission is of particular interest in biological systems, as human tissue is more transparent to red and near infra-red light – potentially allowing such materials to be used in therapeutic settings,13 as well as in OLEDs (organic light emitting diodes) which was originally our entry into such ligand sets.14
As a result, we report herein two such ligands, 2,9-bis(3-trifluoromethylphenyl)-4,7-diphenyl-1,10-phenanthroline and 2,9-bis(4-trifluoromethylphenyl)-4,7-diphenyl-1,10-phenanthroline, and the rhenium(I) carbonyl chloro complexes thereof (illustrated in Scheme 1). We were attracted to the use of the chloro complexes given their desirable photophysical properties as well as the tendency to form air stable complexes.15
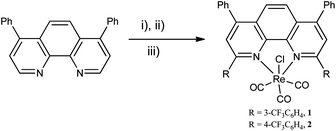 | ||
| Scheme 1 (i) RLi, Et2O–toluene, −78 °C to 0 °C; (ii) H2O, MnO2; (iii) Re(CO)5Cl, toluene, reflux.16 | ||
The cellular uptake of the two complexes in several cell lines has been investigated by fluorescence microscopy, and their cytotoxicity towards a variety of cell lines has been examined by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay.
Complexes 1 and 2 are readily synthesized by heating the ligand and rhenium pentacarbonyl chloride in equimolar quantities in toluene for several hours. Both complexes 1 and 2 can be purified by sublimation under high vacuum between 260 and 280 °C, though complex 2 exhibits decomposition and shows the presence of the free ligand in the products.
Crystals of 1 suitable for analysis by X-ray diffraction were grown at ambient temperature from a saturated solution of 1 in toluene; a thermal ellipsoid projection is shown in Fig. 1 (alternative views are given in the ESI, see Fig. S1–S4†). The structure adopts the expected fac-CO arrangement with a distorted octahedral rhenium centre, where the main distortion occurs as a result of the N,N-chelate bite angle (75.48(1)°). The Re–C and C–O bond lengths are typical of those observed in such fac-tricarbonyl rhenium(I) diimine complexes.8
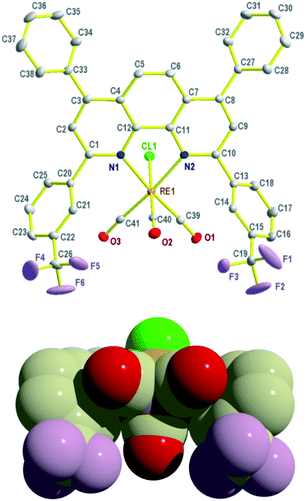 | ||
| Fig. 1 Two views of molecular structure of 1. Selected bond lengths (Å) and angles (°): Re(1)–N(1) 2.213(3), Re(1)–N(2) 2.208(3), Re(1)–Cl(1) 2.4724(9), Re(1)–C(39) 1.924(4), Re(1)–C(40) 1.929(4), Re(1)–C(41) 1.924(4), N(1)–Re(1)–N(2) 75.48(11), Cl(1)–Re(1)–N(1) 83.00(8), Cl(1)–Re(1)–N(2) 81.63(8), Cl(1)–Re(1)–C(40) 177.53(11). | ||
The development of specific cancer drugs depends on a detailed understanding of how the drugs interact with different cell types. We therefore undertook a detailed analysis of the effects of these complexes bearing electron withdrawing groups on different cell types (Table 1). For toxicity screening compounds were dissolved in DMSO (1%), following which the emission shifted to the yellow/green. Given this, we denote the post-DMSO complexes as 1′ and 2′.
| Cell type | Compound 1′ (IC50) | Compound 2′ (IC50) |
|---|---|---|
| a Cells were tested for proliferation after 72 hours using an MTS kit. DMSO (1%) was used as a control in all studies, data represent mean ± SEM of at least three independent experiments done in duplicate. The IC50 was calculated using GraphPad Prism 5 (GraphPad Software), when data was fitted to a nonlinear regression curve. | ||
| A549 | 3.62 ± 0.8 μM | 4.76 ± 2.5 μM |
| THP-1 | 33 ± 16 μM | 14 ± 4.1 μM |
| HeLa | 4.56 ± 3 μM | 83 ± 80 μM |
| CHO | 12.17 ± 6.1 μM | Non-toxic |
| HL60 | Leads to proliferation | Leads to proliferation |
For complexes 1 and 2, the PL spectra are consistent with them acting as red emitters (solution emission for 1 and 2 were 635 nm and 596 nm, respectively, see for example ESI, Fig. S5†cf. about 565 nm for [Re(CO)3(bathophenanthroline sulphate)(py-3-R)] (R = H, CH2OH)11); quantum yield values were 9.7 and 7.2% (ESI, Fig. S6–S8†), respectively. For UV spectra for 1 and 2, see ESI Fig. S9 and S10.†
Both 1′ and 2′ show photoluminescence emission peaks at 451 and 462 nm respectively (ESI, Fig. S11 and S12†). The photoluminescence emission wavelength for both samples reduces in intensity over 21 days at ambient temperature. 1′ shows a reduction in the intensity of the luminescence after 7 days and after 14 shows a change in the shape of the peak with the development of two shoulders at 373 and 415 nm (ESI, Fig. S11†). Complex 2′ shows a reduction in intensity after 3 and 7 days; the luminescence then stabilized from 7 to 21 days (ESI, Fig. S12†).
Depending on the cell types used, the IC50 for 1′ and 2′ differs. Both are particularly toxic for human adenocarcinoma cells (A549), whereas 2′ does not show any toxicity towards CHO cells and is less toxic towards HeLa cells than 1′. Perhaps the most interesting observation of these experiments is that the rhenium complexes 1′ and particularly 2′ encourage the proliferation of HL-60 cells over 72 hours. Preliminary data in 16HBE cell lines also showed that 1′ and 2′ encourage cell growth (ESI, Fig. S13†). We tested both compounds on the monolayer cell line CHO and the suspension cells HL60, and monitored whether or not each can enter cells. The spontaneous fluorescence of the compounds exhibited after activation under a fluorescence microscope was not very bright, but it allowed us to predict cellular up-take of the compounds is indeed occurring in the HL60 cells (ESI, Fig. S14†) (Fig. 2).
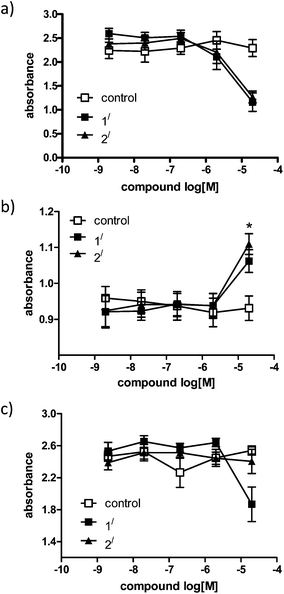 | ||
| Fig. 2 Cell viability assay in (a) A549 cells, (b) HL-60 cells and (c) CHO cells, tested for proliferation after 72 hours using an MTS kit. DMSO used as a control in all studies, data represent mean ± SEM of at least three independent experiments done in duplicate. Absorbance shown is directly proportional to the number of living cells in culture. | ||
We investigated how 1′ and 2′ can induce cell death in the CHO cells and incubated the cells for 24 hours with the compounds and stained with a nucleus and an actin fibre marker (ESI, Fig. S15†).
Neither compounds affected the actin polymerization or the shape and consistence of the nuclei in the cells, i.e. no fragmentation of the nuclei, which can be a sign of early apoptosis, was visible.
These results therefore suggest that neither 1′ nor 2′ induce programmed cell death (apoptosis) in CHO cells, but more work needs to be done to elucidate the actual ways how these compounds kill the cells.
The cytotoxicity of a number of other complexes containing the [fac-Re(CO)3] core has been examined, including phosphine of the form [Re(CO)3(diphosphine)Br].17 For pyridyl or phenanthroline containing complexes, screening against the HeLa cell lines afforded IC50 values (μM) of about 30 for [Re(CO)3(2-appt)Cl] (2-appt = 2-amino-4-phenylamino-6-(2-pyridyl)-1,3,5-triazine)18 and ranging from 17.5–28.5 for a series of thiourea complexes,19a 2.8 to >150 for a series of polypyridyl glucose complexes,19b 3.6 to 40 for a series of polypyridyl florous complexes,19c and 3.6 to >1151.7 for a series of polypyridyl poly(ethylene glycol) complexes.19d
We have attempted to isolate the DMSO complexes 1′ and 2′ using the method of Mayer.20 Our initial attempts however have resulted in oily materials, though in the case of 1, trituration with hexane afforded a yellow/brown glass. The IR spectrum contained a stretch at 950 cm−1 which we tentatively assign to the νS–O stretch of an O-bound DMSO in the complex 1′.21 We note that DMSO complexes bound to Re(CO)3(I) have been previously structurally characterized.22
In summary, we have shown that rhenium(I) tricarbonyl complexes bearing a 2,4,7,9-tetraphenylphenanthroline ligand, where the 2,9-phenyl groups have either meta or para CF3 groups are red emitters. Following treatment with DMSO (1%), the emission is shifted to the yellow/green. In the case of the meta derivative, evaluation against several cell lines revealed anti-cancer activity against HeLa and A549 cell lines; the para derivative caused proliferation of HL-60 cells, and toxicity towards A549 cells. Further work is in progress to determine the exact nature of 1′ and 2′, and also the cytotoxicity profiles of the meta- and para-CF3 ligands described herein in combination with other metals.
The Big C Cancer Appeal and the Whilelaw Frater Cancer Trust are thanked for financial support. Dr Kevin Welham (University of Hull) is thanked for Mass Spectroscopy on 1′.
Notes and references
- L. J. K. Boerner and J. M. Zaleski, Curr. Opin. Chem. Biol., 2005, 47, 5282 Search PubMed.
- See for example (a) J. Martinez-Lillo, T. F. Mastropietro, R. Lappano, A. Madeo, M. E. Alberto, N. Russo, M. Maggiolini and G. De Munno, Chem. Commun., 2011, 47, 5283 RSC; (b) P. S. Donnelly, Dalton Trans., 2011, 40, 999 RSC.
- K. A. Stephenson, S. R. Banerjee, T. Besanger, O. O. Sogbein, M. K. Levadala, N. McFarlane, J. A. Lemon, D. R. Boreham, K. P. Maresca, J. D. Brennan, J. W. Babich, J. Zubieta and J. F. Valliant, J. Am. Chem. Soc., 2004, 126, 8598 CrossRef CAS PubMed.
- See for example M. J. Edelman, G. Clamon, D. Kahn, M. Magram and B. R. Line, J. Clin. Oncol., 2007, 25, 7672 Search PubMed.
- (a) R. Alberto, R. Schibli, R. Waibel, U. Abram and A. P. Schubiger, Coord. Chem. Rev., 1999, 190–192, 901 CrossRef CAS; (b) K. K.-W. Lo, K. Y. Zhang and S. P.-Y. Li, Eur. J. Inorg. Chem., 2011, 3551 CrossRef CAS.
- (a) L. Wei, J. W. Babich, W. Ouellette and J. Zubieta, Inorg. Chem., 2006, 45, 3057 CrossRef CAS PubMed; (b) K. K.-W. Lo, M.-W. Louie and K. Y. Zhang, Coord. Chem. Rev., 2010, 254, 2603 CrossRef CAS PubMed; (c) R. G. Balasingham, M. P. Coogan and F. L. Thorp-Greenwood, Dalton Trans., 2011, 40, 11663 RSC; (d) K. K.-W. Lo, K. Y. Zhang and S. P.-Y. Li, Eur. J. Inorg. Chem., 2011, 3551 CrossRef CAS; (e) K. K.-W. Lo, A. W.-T. Choi and W. H.-T. Law, Dalton Trans., 2012, 41, 6021 RSC.
- Z. Si, J. Li, B. Li, Z. Hong, S. Lu, S. Lui and W. Li, Appl. Phys. A, 2007, 88, 643 CrossRef CAS.
- See for example, (a) M. S. Wrighton and D. L. Morse, J. Am. Chem. Soc., 1974, 96, 998 CrossRef CAS; (b) P. J. Giordano, S. M. Fredericks, M. S. Wrighton and D. L. Morse, J. Am. Chem. Soc., 1978, 100, 2257 CrossRef CAS; (c) P. J. Giordano and M. S. Wrighton, J. Am. Chem. Soc., 1979, 101, 2888 CrossRef CAS; (d) S. M. Fredericks, J. C. Luong and M. S. Wrighton, J. Am. Chem. Soc., 1979, 101, 7415 CrossRef CAS; (e) W. K. Smothers and M. S. Wrighton, J. Am. Chem. Soc., 1983, 105, 1067 CrossRef CAS; (f) L. Wallace, D. C. Jackman, D. P. Rillema and J. W. Merkert, Inorg. Chem., 1995, 34, 5210 CrossRef CAS; (g) D. J. Stufkens and A. Vlček Jr, Coord. Chem. Rev., 1998, 177, 127 CrossRef CAS; (h) A. Vlček Jr, Coord. Chem. Rev., 2000, 200, 933 CrossRef; (i) J. Dyer, W. J. Blau, C. G. Coates, C. M. Creely, J. D. Gavey, M. W. George, D. C. Grills, S. Hudson, J. M. Kelly, P. Matousek, J. J. McGarvey, J. McMaster, A. W. Parker, M. Towrie and J. A. Weinstein, Photochem. Photobiol. Sci., 2003, 2, 542 RSC; (j) M. K. Kuimova, X. Z. Sun, P. Matousek, D. C. Grills, A. W. Parker, M. Towrie and M. W. George, Photochem. Photobiol. Sci., 2007, 6, 1158 RSC; (k) A. Cannizzo, A. M. Blanco-Rodriguez, A. E. Nahhas, J. Šebera, S. Záliš, A. Vlček Jr and M. Chergui, J. Am. Chem. Soc., 2008, 130, 8967 CrossRef CAS PubMed; (l) A. E. Nahhas, C. Consani, A. M. Blanco-Rodriguez, K. M. Lancaster, O. Braem, A. Cannizzo, M. Towrie, I. P. Clark, M. Chergui, S. Záliš and A. Vlček Jr, Inorg. Chem., 2011, 50, 2932 CrossRef PubMed; (m) M.-W. Louie, T. T.-H. Fong and K. K.-W. Lo, Inorg. Chem., 2011, 50, 9465 CrossRef CAS PubMed.
- (a) E. M. Kober, B. P. Sullivan, W. J. Caspar and T. Meyer, J. Am. Chem. Soc., 1980, 102, 7383 CrossRef CAS; (b) E. M. Kober, J. L. Marshall, W. J. Dressick, B. P. Sullivan, J. V. Caspar and T. Meyer, Inorg. Chem., 1985, 24, 2755 CrossRef CAS.
- D. V. Partyka, N. Deligonul, M. P. Washington and T. G. Gray, Organometallics, 2009, 28, 5837 CrossRef CAS.
- A. J. Amoroso, M. P. Coogan, J. E. Dunne, V. Fernández-Moreira, J. B. Hess, A. J. Hayes, D. Lloyd, C. Millet, S. J. A. Pope and C. Williams, Chem. Commun., 2007, 3066 RSC.
- A. J. Hallet and S. J. A. Pope, Inorg. Chem. Commun., 2011, 14, 1606 CrossRef PubMed.
- J. V. Frangioni, Curr. Opin. Chem. Biol., 2003, 7, 626 CrossRef CAS PubMed.
- S. E. Watkins and V. Christou, Rhenium compounds, WO03079737, 2003.
- W. Liu, J. Xiong, Y. Wang, X.-H. Zhou, R. Wang, J.-L. Zuo and X.-Z. You, Organometallics, 2009, 28, 755 CrossRef CAS.
- The ligand preparation was adapted from C. O. Dietrich-Buchecker, P. A. Marnot and J. P. Sauvage, Tetrahedron Lett., 1982, 23, 5291 CrossRef CAS.
- J. Zhang, J. J. Vittal, W. Henderson, J. R. Wheaton, I. H. Hall, T. S. A. Hor and Y. K. Yan, J. Organomet. Chem., 2002, 650, 123 CrossRef CAS.
- D.-L. Ma, C.-M. Chi, F.-M. Siu, M. Yang and K.-Y. Wong, Inorg. Chem., 2007, 46, 740 CrossRef CAS PubMed.
- (a) K. K.-W. Lo, M.-W. Louie, K.-S. Sze and J. S.-Y. Lau, Inorg. Chem., 2008, 47, 602 CrossRef CAS PubMed; (b) M.-W. Louie, H.-W. Liu, M. H.-C. Lam, Y.-W. Lam and K. K.-W. Lo, Chem.–Eur. J., 2011, 17, 8304 CrossRef CAS PubMed; (c) M.-W. Louie, A. W.-T. Choi, H.-W. Liu, B. T.-N. Chan and K. K.-W. Lo, Organometallics, 2012, 31, 5844 CrossRef CAS; (d) A. W.-T. Choi, M.-W. Louie, S. P.-Y. Li, H.-W. Lei, B. T.-N. Chan, T. C.-Y. Lam, A. C.-C. Lin, S.-H. Cheng and K. K.-W. Lo, Inorg. Chem., 2012, 51, 13289 CrossRef CAS PubMed.
- D. D. DuMez and J. M. Mayer, Inorg. Chem., 1995, 34, 6396 CrossRef CAS.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley, New York, 3rd edn, 1978 Search PubMed.
- M. Casanova, E. Zangrando, F. Munini, E. Iengo and E. Alessio, Dalton Trans., 2006, 5033 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterisation of 1 and 2; alternative views of 1; PL spectra of 1, 2, 1′ and 2′; cell assays for 1′ and 2′. CCDC 755744. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra43207f |
| This journal is © The Royal Society of Chemistry 2013 |
