A microfluidic device for quantitative analysis of chemoattraction in plants
Mitsuhiro Horade†a, Masahiro M. Kanaoka*b, Motoki Kuzuyab, Tetsuya Higashiyamaabc and Noritada Kaji‡*a
aJST, ERATO, Higashiyama Live-Holonics Project, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, Aichi 464-8602, Japan. E-mail: kaji.noritada@g.mbox.nagoya-u.ac.jp
bDivision of Biological Science, Graduate School of Science, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, Aichi 464-8602, Japan. E-mail: mkanaoka@bio.nagoya-u.ac.jp
cWPI-ITbM, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, Aichi 464-8602, Japan
First published on 2nd October 2013
Abstract
To precisely quantitate the effect of chemoattractants on directional pollen tube growth, a new microdevice was developed. Torenia fournieri pollen tubes, which generally grow freely on agarose medium, were funneled through a narrow flow channel that splits into a T-shaped channel leading to two reservoirs. The main channel was thus divided in two so that pollen tubes could choose their growth in either the left or right direction. Liquid solution or plant tissues were loaded into the reservoir, and diffusible molecules from the materials gradually spread in the narrow channel, leading to a concentration gradient. When egg-cell containing ovules were placed in one reservoir, pollen tubes grew selectively in that direction, suggesting that materials secreted from the ovules attracted the pollen tubes. Furthermore, UV-irradiation of female gametophytes in ovules decreased their ability to attract pollen tube growth. These results suggest that this novel device provides a unique platform for screening materials that may attract pollen tubes and for quantitatively analyzing the chemical features of attractants.
Introduction
Male and female gametes of higher plants are generated in spatially separated tissues. The male gametes, sperm cells, are produced inside pollen grains, and the female gametophyte is embedded in the ovule of the pistil. Sperm cells are immobile and are carried to the female gametophyte by a tip-growing cell, the pollen tube, which emerges from the pollen grain. After germinating on the stigma, the pollen tube travels a long distance through the pistil, including the style and ovary, and then enters into the female gametophyte through the micropyle of the ovule to achieve fertilization. During this journey, only compatible pollen tubes are selectively attracted and reach their final destination.1 Therefore, successful fertilization relies on the precise interaction between the male and female tissues.Genetic and physiological evidence suggests that the direction of pollen tube growth is regulated by female tissue-derived molecular signals.2,3 Arabidopsis mutants defective in female gametophyte development or in the expression of female gametophyte-specific genes cause the misguidance of pollen tubes.2,4–9 Many proteins are specifically expressed and secreted from pistil tissues and the female gametophyte along the path of pollen tube growth, and these would be candidates for novel pollen tube attractants.10,11 Because the female gametophyte is located deep inside the pistil, it is difficult to observe pollen tube growth in vivo in real time. Thus, most attempts to identify materials with pollen tube-attraction activities have been conducted under in vitro or semi-in vitro conditions.12–17 Classical studies have suggested that the contribution of calcium ions and female tissues are necessary for pollen tube attraction.14 More recently, it is reported that gamma-amino butyric acid (GABA) can stimulate pollen tube growth in vitro.16 Disruption of the Arabidopsis POP2 gene, which encodes a transaminase that degrades GABA, caused a disturbance of the GABA gradient in the pistil, leading to growth arrest and misguidance of pollen tubes.14 Recently, two defensin-like cysteine-rich proteins, LURE1 and LURE2, which are secreted from the synergid cells of the female gametophyte, have been shown to function as pollen tube attractants in Torenia.15 The attraction of pollen tubes by LUREs is strictly regulated; only pollen tubes of the same species can be attracted effectively, and only at the appropriate concentrations of LUREs.3,18
Previous studies have suggested that the reorientation of pollen tube growth is controlled by a gradient of attractant molecules.15,19 Pollen tubes grown in in vitro culture medium tend to grow toward the source of an attractant, and the attraction rate is influenced by the concentration of the attractant. Most attraction assays have been performed under a bulk condition in which the attractants are spotted on medium that is spread radially. This makes it difficult to determine the concentration of attractant molecules at the pollen tube. Generally, pollen tubes grown on agarose medium show a wavy growth pattern even in the absence of an attractant. Thus, meticulous observations are required to distinguish between attracted pollen tubes and randomly growing pollen tubes.
To overcome these difficulties in evaluating pollen tube attraction, we aimed to develop a microdevice that would enable quantitative chemical stimulation for pollen tube attraction. Recently, the development of microdevices for plant assay systems has been reported.20–22 To study the physiology of growing Arabidopsis thaliana roots, the “root on a chip” platform allows high-precision chemical stimulation of particular cells within the roots, at a spatial resolution of 10–800 μm.21 The root chip has enabled large-scale phenotyping of root metabolism and signaling, as reported by Quake et al.22 Subsequently, a “pollen tube on a chip,” first reported by Zohar et al. in 2011, was developed for studies of pollen tube guidance.20 In contrast to roots, which have diameters of about 75 μm and growth rates of 140–160 μm h−1, pollen tubes have a 20 μm diameter and growth rates of 200–400 μm h−1. Thus, more gentle and precise operations must be performed on the chip.
Here, we report the development of a novel microfluidic channel-based pollen tube assay system using a biocompatible polydimethylsiloxane (PMDS) chip. We designed a device with narrow microfluidic channels to restrict the growth path of pollen tubes. Connected to the neck of the main channel, there are two outlet wells (reservoirs) into which various materials can be applied, allowing pollen tube attraction to be examined by monitoring the tube growth direction under precisely defined concentration gradients of chemoattractants. These two factors, how to orient the emerged pollen tubes into the concentration gradients of chemoattractants and generate chemical gradients over an extended time period, are major focuses of this research to overcome the past experimental system. Compared with the previously reported microdevice, our device is simple and easy to handle, and has a broad range of potential applications for future assay systems.
Experimental
Design and fabrication of the device
The polydimethylsiloxane (PDMS, Sylgard® 184; Dow Corning Corp.) devices for the pollen tube attraction assay were fabricated using a soft lithography technique.23 Each device consisted of a T-shaped flow channel (100 μm in height and 100–1000 μm in width), an inlet well into which a pollinated style can be inserted, and two outlet wells (reservoirs) into which various materials can be applied (Fig. 1a). The devices were designed using Auto CAD software (Autodesk) and reproduced onto transparency film. The flow channel features, with a height of 100 μm, were fabricated using SU-8 photoresist (SU-8 3050; Microchem Corp.) on a 4-inch silicon wafer (Fig. 1b and c). After the construct was baked for 45 min at 95 °C, the SU-8 photoresist was exposed to UV light (250 mJ cm−2) using a mask aligner (MJB4, SUSS MicroTec KK), baked for another 5 min at 95 °C, and developed by SU-8 developer (Fig. 1d and e). For device fabrication, a PDMS mixture of base and curing agent with a weight ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was poured onto the mold located in a polystyrene Petri dish, degassed for 20–40 min in a vacuum desiccator, and cured for 2 h at 90 °C. The PDMS replica was then peeled off of the mold (Fig. 1f). Finally, holes of 1.5 mm diameter for an inlet well and two reservoirs were punched in the PDMS device (Fig. 1g).
1 was poured onto the mold located in a polystyrene Petri dish, degassed for 20–40 min in a vacuum desiccator, and cured for 2 h at 90 °C. The PDMS replica was then peeled off of the mold (Fig. 1f). Finally, holes of 1.5 mm diameter for an inlet well and two reservoirs were punched in the PDMS device (Fig. 1g).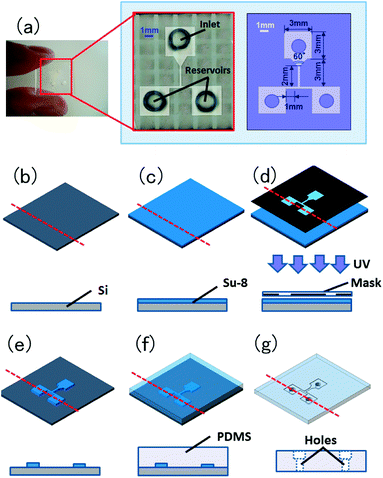 | ||
| Fig. 1 Design of the pollen tube growth device. (a) A macroscopic photo and schematics of the microfluidic device. This device consists of a flow channel (100 μm in height and 100–1000 μm in width) with a three-way intersection, an inlet well into which the style of a pollinated pistil is placed, and two outlet wells (reservoirs) into which various compounds may be applied. (b–g) Birds-eye and cross-section schematics of the device fabrication process. (b and c) SU-8 photoresist (100 μm thickness) is spin-coated onto a silicon wafer. (d) SU-8 photoresist is exposed to UV light utilizing a mask aligner and mask-designed device pattern. (e) SU-8 photoresist is developed by the SU-8 developer for 20 min, and isopropyl alcohol is applied for 10 s. (f) Liquid PDMS is poured onto the SU-8 master in a polystyrene Petri dish, this is baked at 90 °C for 1–2 h using a hotplate, and the PDMS mold is peeled off of the SU-8 master. (g) Holes (1.5 mm diameter) for an inlet well and two reservoirs are punched in the PDMS mold. | ||
Preparation of the pollen tube assay device
To prepare the pollen tube growth medium, ultra-low melting point agarose (Type IX-A; Sigma) was added at a concentration of 1.5% (w/v) to modified Nitsche medium,24 and the medium was heated to 96 °C to melt the agarose. The PDMS device was placed upside down in the center of a glass-bottom dish (Matsunami), and approximately 20 μl of the medium were injected from the inlet well of the device using a micropipette to fill the area between the PDMS device and the glass. The medium was allowed to solidify at 4 °C, and the device was stored until use.Plant materials and pollen tube culture
Torenia fournieri cv. blue and white plants were grown under ambient conditions.18 A mature pistil was hand-pollinated and then cut at the junction between the style and ovary. The cut style was carefully placed in the assay device, with the cut end completely in contact with the pollen tube growth medium. An ovary from a mature T. fournieri flower or control buffer was placed in the reservoir through a pit on the reservoir. The glass bottom dish containing the device was kept moist in an incubation chamber at 25 °C for 12 to 16 h to allow the pollen tubes to grow.Observation of pollen tubes
The growth of pollen tubes was observed under a conventional bright field microscope (Olympus IX71; Olympus). The number of pollen tubes growing in the flow channel was counted by eye.Diffusion simulation in the flow channel
To confirm that the appropriate concentration gradient of materials remains in the flow channel, the time dependence of the concentration gradient was estimated theoretically by the Finite Element Method using commercial software (CoventorWare; Coventor, Inc.). For the simulation conditions, a solution containing 1 μM test compound was applied continuously to one of the two reservoirs. The diffusion coefficient was set at 100 μm2 s−1, which is representative of the diffusion of a small secreted protein with a molecular mass of 10 kDa.Visualization in the flow channel
To monitor the diffusion of materials from the reservoir, 10 μl of 1 μM Alexa dye-conjugated 10 kDa dextran was applied to one of the two reservoirs. The Alexa 488 and Alexa 546 fluorophores were excited by a UV lamp with blue and green filters, respectively, and the fluorescence was observed under fluorescence microscope (IX71; Olympus). Alternatively, Alexa 488 was excited by a 488 nm argon laser, and the fluorescence was observed under a confocal microscope (LSM780; Carl Zeiss).Data analysis
Fluorescence intensity was calculated using ImageJ (NIH) or ZEN2009 software (Carl Zeiss).Results and discussion
Design of a pollen-tube growth assay microdevice
The structure of the microdevice is shown in Fig. 1a. There is an open end designed for placement of a pollinated cut style, allowing the pollen tubes to grow on the medium in the device. Because pollen tubes tend to grow with random orientations just after emergence from the cut end of a style, the entrance narrows toward the neck flow channel to gather as many pollen tubes as possible in the neck flow channel. The neck flow channel was designed to mimic the style and the top part of the ovary and thus ends as a narrow straightaway. The other side of the neck flow channel connects to a horizontal flow channel placed perpendicularly, allowing the pollen tubes to grow toward either the left or right side. Finally, the horizontal flow channel is connected at each end to a reservoir, where materials to be tested can be placed. Because the pollen tube growth medium fully covers the space between the cover glass and the PDMS resin, the medium in the device does not dry out during the assay. The biocompatibility between Torenia fournieri pollen tubes and PDMS is good, and pollen tube growth in the device was identical to that on agarose gel medium, i.e., 200–400 μm h−1 (data not shown).With the previous device design,20 the style and ovules were placed in an open microchannel and then enclosed by a pollen growth medium layer. It is extremely difficult to place ovules by hand such that they are embedded in the chamber (250 × 250 μm2) with their micropyles facing the chamber opening to the main channel, without injuring the ovules. Even after enclosing the style and ovules in a layer of pollen growth medium, chemoattractants from ovules may diffuse easily from the chamber to the bottom of the medium layer and across the PDMS wall regions. Therefore, the microdevice could work as a guide for pollen tube growth, but would not produce a chemical concentration gradient.
Optimization of the flow channel design for chemoattraction
The design of the microdevice was optimized for pollen tube entrance to the flow channel and for lane width of the flow channel (Fig. 2a). Two different designs for pollen tube entrance were tested: one was T-shaped, and the other was V-shaped. In the T-shaped design (Fig. 2b), the flow channel is narrow from the start, and most of the pollen tubes emerging from the cut end of the style were trapped at this step, with only a few pollen tubes entering the neck flow channel. In the V-shaped design, the entrance is wider and gradually narrows to the final width of the neck flow channel (Fig. 2c). With the V-shaped entrance, most of the pollen tubes were gathered smoothly at the connection between the entrance and the neck flow channel. Thus, the latter configuration was chosen for the microdevice entrance.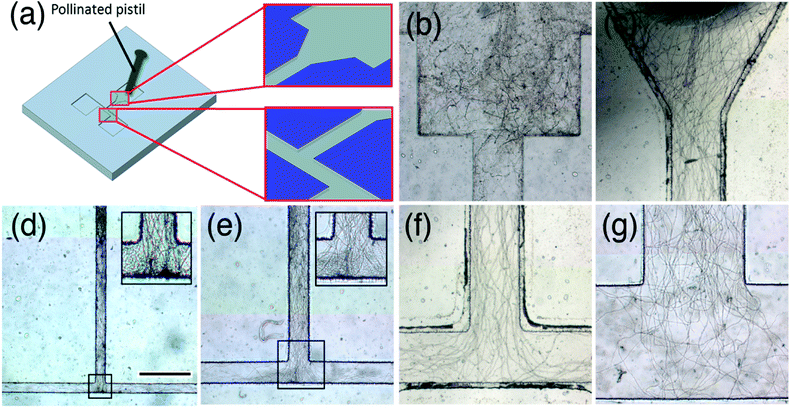 | ||
| Fig. 2 Evaluation of the flow channel shape and width. (a) Schematic of the microfluidic device. A pollinated pistil that is cut at the junction between the style and ovary is placed in the inlet such that pollen tubes can emerge from the cut end of the style and enter the flow channel. The optimal shape of the flow channel entrance and optimal width of the flow channel were investigated. Comparison of pollen tube growth between (b) a “step-down” entrance and (c) a V-shaped entrance. Comparison of pollen tube growth in channels with widths of (d) 100 μm, (e) 200 μm, (f) 500 μm, (g) 1000 μm. Inlets in (d) and (e) are higher magnification images of boxed regions. The scale bar shown in (d) is 500 μm and applies to (b–g). | ||
Next, the optimum lane width of the flow channel was determined. Because the style of T. fournieri is about 1 mm in diameter and pollen tubes grow in its central region (approximately 300 μm), it was necessary to mimic the pollen tube path to the ovary. Several devices were assembled with channel lane widths of 100, 200, 500, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μm. In all cases, pollen tubes grew well in the flow channel (Fig. 2d–g). However, with the 100 and 200 μm channel lane widths, the pollen tubes were too crowded, making it difficult to count the pollen tubes and to track the path of each (Fig. 2d and e). With the 1
000 μm. In all cases, pollen tubes grew well in the flow channel (Fig. 2d–g). However, with the 100 and 200 μm channel lane widths, the pollen tubes were too crowded, making it difficult to count the pollen tubes and to track the path of each (Fig. 2d and e). With the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μm channel width, random waving or curving of the pollen tubes was observed in the flow channel, making it difficult to distinguish between random growth and oriented growth (Fig. 2g). The 500 μm channel width provided the best results; the pollen tubes grew straight and were easily discernible (Fig. 2f). Therefore, the 500 μm channel lane width was chosen for the chemoattraction assay.
000 μm channel width, random waving or curving of the pollen tubes was observed in the flow channel, making it difficult to distinguish between random growth and oriented growth (Fig. 2g). The 500 μm channel width provided the best results; the pollen tubes grew straight and were easily discernible (Fig. 2f). Therefore, the 500 μm channel lane width was chosen for the chemoattraction assay.
Formation of a concentration gradient in the flow channel
Prior to experiments using the 500 μm channel width, a diffusion simulation experiment was conducted with 10 kDa material placed in the reservoir (Fig. 3a). At least 3 h were required for the material to reach the three-way intersection of the T-shaped flow channel (Fig. 3b and c). Furthermore, its concentration at the three-way intersection of the T-shaped flow channel formed a gradient that was maintained for 10 h (Fig. 3b and c). Time-lapse microscopy was used to monitor the diffusion of Alexa-conjugated dextran in the flow channel (Fig. 3e and f). The fluorescent signal spread with time, again reaching the three-way intersection of the T-shaped flow channel in approximately 3 h (Fig. 3e). Thus, one advantage of this microdevice is that a chemical gradient based on the diffusion constant of the applied material can be generated easily and reproducibly and remains long enough to enable pollen tubes to reach it. By applying different materials to each reservoir, two chemical gradients could be formed simultaneously at the three-way intersection (Fig. 3f and g).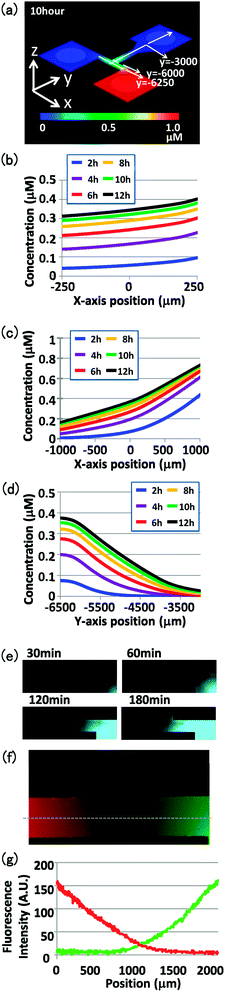 | ||
Fig. 3 Concentration gradient in the flow channel. (a) Schematic of the diffusion simulation results with the 500 μm channel width, showing the concentration gradient of 10 kDa material in the flow channel at 10 h after application. Concentration changes over time were calculated at (b) the entrance of the intersection (y = −6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), (c) the center of the intersection (y = −6 000), (c) the center of the intersection (y = −6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250), and (d) the intersection (x = 0) in the flow channel. (e) Changes in fluorescence in the flow channel after loading Alexa 488-conjugated 10 kDa dextran to a reservoir. Images were acquired at 30, 60, 120, and 180 min after loading the material (left to right). (f) Fluorescence gradient in the flow channel after loading Alexa 546-conjugated 10 kDa dextran (red) in the left reservoir and Alexa 488-conjugated 10 kDa dextran (green) in the right reservoir. The image was acquired 3 h after loading the materials. (g) Fluorescence gradient of Alexa 546 (red) and Alexa 488 (green) at the flow channel intersection (dotted line in f). Fluorescence intensities were evaluated using ImageJ software. 250), and (d) the intersection (x = 0) in the flow channel. (e) Changes in fluorescence in the flow channel after loading Alexa 488-conjugated 10 kDa dextran to a reservoir. Images were acquired at 30, 60, 120, and 180 min after loading the material (left to right). (f) Fluorescence gradient in the flow channel after loading Alexa 546-conjugated 10 kDa dextran (red) in the left reservoir and Alexa 488-conjugated 10 kDa dextran (green) in the right reservoir. The image was acquired 3 h after loading the materials. (g) Fluorescence gradient of Alexa 546 (red) and Alexa 488 (green) at the flow channel intersection (dotted line in f). Fluorescence intensities were evaluated using ImageJ software. | ||
Pollen tube growth on the micro-device
As a control experiment, we monitored pollen tube growth behavior in the medium. We used T. fournieri pollen because its pollen tubes grow well in a modified Nitsche medium and are attracted to conspecific ovules in that medium.18,25 When a pollinated T. fournieri style was cut and placed on the growth medium, pollen tubes emerged from the cut end of the style and continued to grow in the medium. In the absence of female tissues as a source of attractants, pollen tube growth in the medium was random (Fig. 4b). When pollen tubes were cultured in medium containing an ovary, which contains about 200–300 mature ovules, their growth was directed toward the ovary immediately after emerging from the cut end of the style (Fig. 4c). This suggests that the growth orientation of pollen tubes is regulated by diffusible signaling molecules from the ovary and that the effective distance of the attractant is more than 3 mm from the ovary.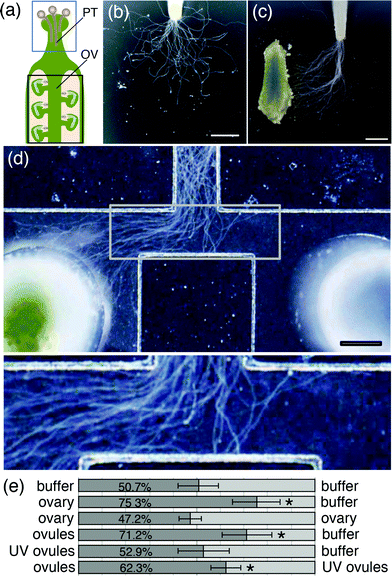 | ||
| Fig. 4 Pollen tube growth assay on agarose gel and in the microdevice. (a) Schematic of the pistil of a typical flowering plant. Pollen tubes (PT) grow through the style of the pistil toward the ovary (OV). The blue box outlines a cut style, which was placed on agarose medium or in the microdevice. The black box outlines an ovary, which was used in the pollen tube attraction assay. Pollen tube growth was examined (b and c) on agarose gel and (d and e) in the device with a 500 μm channel width. (b) In the absence of an ovary, pollen tubes grew in a random pattern on agarose gel. (c) In the presence of an ovary (see to the left), pollen tube growth on agarose gel was oriented toward the ovary. (d) In the microdevice, pollen tube growth was oriented toward an ovary placed in the left reservoir. The bottom panel shows an image at higher magnification. (e) Summary of pollen tube attraction assays using the microdevice. Materials placed in the left or right reservoir are shown on the left or right side of the bar graph, respectively. The numbers on the dark gray bars represent the average percentage of pollen tubes oriented toward the left reservoir. At least five assays were performed for each experimental condition. Asterisks indicate significant differences compared with the negative control (p < 0.01, Student's t-test). Scale bars represent 1 mm in (b and c) and 500 μm in (d). | ||
Using the microdevice, we tested whether pollen tube growth can be regulated by molecules from an ovary placed in the reservoir (Fig. 4d and e). First, pollinated T. fournieri pistils were cut, placed at the top end of the microdevice, and incubated without any material in the reservoirs; there was no significant difference between the numbers of pollen tubes growing to the left versus the right flow channel at the T-junction (Fig. 4e). Next, at the same time the pollinated style was placed in the device, an ovary was placed in the left reservoir; the right reservoir was kept empty. The pollen tubes, which grow at a rate of about 200–400 μm h−1, reached the T-junction of the flow channel at 5–10 h after pollination, by which time a concentration gradient had been established at the T-junction, based on the diffusion rate of material from the reservoir. In contrast to pollen tube growth in the absence of an ovary (Fig. 4e), significant numbers of the pollen tubes were oriented toward the left flow channel (Fig. 4d and e, p < 0.01, Student's t-test). As expected, most of the pollen tubes were oriented toward the right flow channel when an ovary was placed in the right reservoir (data not shown). When ovaries were placed in both the left and right reservoirs, there was no significant difference between the numbers of pollen tubes growing to the left versus right flow channel, suggesting that diffusion occurred similarly from both flow channels (Fig. 4e, p < 0.77, Student's t-test).
We then investigated which part of the ovary influences this attraction. When ovules were taken from the placenta and 10–20 ovules were placed in the left resorvoir, pollen tubes were significantly attracted to the ovules (Fig. 4e, p < 0.01, Student's t-test).When the female gametophytes were destroyed by UV irradiation and these ovules (UV ovules) were applied to the left reservoir, pollen tube growth showed no orientation toward the left channel (Fig. 4e). When normal ovules and UV ovules were applied to the left and right reservoir, respectively, pollen tubes were significantly oriented to the left (Fig. 4e, p < 0.01, Student's t-test). These results suggest that the female gametophyte is essential for pollen tube attraction.
Some chemoattractant proteins also affect growth rate of pollen tubes.13 Therefore, it is important to distinguish growth stimulation and attraction.26 As shown in Fig. 4c, pollen tubes that are attracted to the ovary start to change their growth orientation at random position in the bulk condition; some pollen tubes changed their direction just after the emergence from the style, and others grew straight for a while and later changed their direction. In contrast, all the pollen tubes change their direction at T-junction in the device, so it was easy to measure their growth rate. The growth rate of attracted pollen tubes was 13.4 ± 4.8 μm min−1 (n = 20) and that of unattracted pollen tubes was 16.3 ± 2.5 μm min−1 (n = 10). These rates were not significantly different (Student's t-test, p = 0.098). Taken together, these results suggested that pollen tube attraction is the regulation of growth orientation but not the stimulation of growth, and the developed microdevice could be very useful for the quantitative analysis of pollen tube attraction.
Conclusions
Here, we developed a microdevice with optimized flow channel design and width for use in studies of pollen tube growth and attraction. As pollen tubes usually grow through a narrow region of the style tissue, typically less than 500 μm in diameter for hollow styles, or through the intercellular space in the transmitting tract of the pistil, a flow channel with a width of 500 μm, mimicking the natural conditions in plants, was found to be optimal for the growth of pollen tubes. In the absence of attractant tissue, pollen tubes grew toward both sides of the flow channel, similar to the random growth pattern observed on agarose plates. In the presence of ovules, most of the pollen tubes grew toward the reservoir containing ovules. Thus, molecules derived from female gametophytes may be candidates for pollen tube attractants. Compared to earlier devices,20 our novel microdevice allows the formation of a gradient of materials that diffuse from each reservoir and enables a clear “left or right” distinction in pollen tube growth orientation. Thus, this device is suitable for the generation of a constant concentration gradient of diffusible molecules, and for the observation, quantitative analysis and high-throughput analysis of pollen tube attraction.Acknowledgements
We thank Dr Yuki Hamamura for time-lapse imaging of concentration gradient in the flow channel and Ms Naoko Iwata for plant care, Dr Daniel P. Matton and Dr Hideyuki Arata for critical reading and comments. This work was supported by the Exploratory Research for Advanced Technology (ERATO), Japan Science and Technology Agency (JST) and the grants from the Japan Society for the Promotion of Science (23012021, 24113510, and 24570045 to M.M.K., 24760101 to M.H.)References
- K. K. Shimizu, Popul. Ecol., 2002, 44, 0221–0233 CrossRef.
- T. Higashiyama, Plant Cell Physiol., 2010, 51, 177–189 CrossRef CAS PubMed.
- H. Takeuchi and T. Higashiyama, PLoS Biology, 2012, 10, e1001449 CAS.
- Y. H. Chen, H. J. Li, D. Q. Shi, L. Yuan, J. Liu, R. Sreenivasan, R. Baskar, U. Grossniklaus and W. C. Yang, Plant Cell, 2007, 19, 3563–3577 CrossRef CAS PubMed.
- J. M. Escobar-Restrepo, N. Huck, S. Kessler, V. Gagliardini, J. Gheyselinck, W. C. Yang and U. Grossniklaus, Science, 2007, 317, 656–660 CrossRef CAS PubMed.
- M. Hulskamp, K. Schneitz and R. E. Pruitt, Plant Cell, 1995, 7, 57–64 Search PubMed.
- R. D. Kasahara, M. F. Portereiko, L. Sandaklie-Nikolova, D. S. Rabiger and G. N. Drews, Plant Cell, 2005, 17, 2981–2992 CrossRef CAS PubMed.
- K. K. Shimizu and K. Okada, Development, 2000, 127, 4511–4518 CAS.
- L. K. Wilhelmi and D. Preuss, Science, 1996, 274, 1535–1537 CrossRef CAS.
- M. W. Jones-Rhoades, J. O. Borevitz and D. Preuss, PLoS Genetics, 2007, 3, 1848–1861 CAS.
- C. W. Tung, K. G. Dwyer, M. E. Nasrallah and J. B. Nasrallah, Plant. Physiol., 2005, 138, 977–989 CrossRef CAS PubMed.
- J. P. Mascarenhas and L. Machlis, Nature, 1962, 196, 292–293 CrossRef CAS.
- A. Y. Cheung, H. Wang and H. M. Wu, Cell, 1995, 82, 383–393 CrossRef CAS.
- R. Palanivelu, L. Brass, A. F. Edlund and D. Preuss, Cell, 2003, 114, 47–59 CrossRef CAS.
- S. Okuda, H. Tsutsui, K. Shiina, S. Sprunck, H. Takeuchi, R. Yui, R. D. Kasahara, Y. Hamamura, A. Mizukami, D. Susaki, N. Kawano, T. Sakakibara, S. Namiki, K. Itoh, K. Otsuka, M. Matsuzaki, H. Nozaki, T. Kuroiwa, A. Nakano, M. M. Kanaoka, T. Dresselhaus, N. Sasaki and T. Higashiyama, Nature, 2009, 458, 357–361 CrossRef CAS PubMed.
- S. Kim, J. C. Mollet, J. Dong, K. Zhang, S. Y. Park and E. M. Lord, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 16125–16130 CrossRef CAS PubMed.
- H. Wang, L. C. Boavida, M. Ron and S. McCormick, Plant Cell, 2008, 20, 3300–3311 CrossRef CAS PubMed.
- M. M. Kanaoka, N. Kawano, Y. Matsubara, D. Susaki, S. Okuda, N. Sasaki and T. Higashiyama, Ann. Bot., 2011, 108, 739–747 CrossRef CAS PubMed.
- H. Goto, S. Okuda, A. Mizukami, H. Mori, N. Sasaki, D. Kurihara and T. Higashiyama, Plant Cell Physiol., 2011, 52, 49–58 CrossRef CAS PubMed.
- A. K. Yetisen, L. Jiang, J. R. Cooper, Y. Qin, R. Palanivelu and Y. Zohar, J. Micromech. Microeng., 2011, 21, 054018 CrossRef.
- M. Meier, E. M. Lucchetta and R. F. Ismagilov, Lab Chip, 2010, 10, 2147–2153 RSC.
- G. Grossmann, W. J. Guo, D. W. Ehrhardt, W. B. Frommer, R. V. Sit, S. R. Quake and M. Meier, Plant Cell, 2011, 23, 4234–4240 CrossRef CAS PubMed.
- J. R. Anderson, D. T. Chiu, R. J. Jackman, O. Cherniavskaya, J. C. McDonald, H. Wu, S. H. Whitesides and G. M. Whitesides, Anal. Chem., 2000, 72, 3158–3164 CrossRef CAS.
- T. Higashiyama, H. Kuroiwa, S. Kawano and T. Kuroiwa, Plant Cell, 1998, 10, 2019–2032 CAS.
- S. Okuda, T. Suzuki, M. M. Kanaoka, H. Mori, N. Sasaki and T. Higashiyama, Mol. Plant, 2013, 6, 1074–1090 CrossRef CAS PubMed.
- W. M. Lush, Trends Plant Sci., 1999, 4, 413–418 CrossRef.
Footnotes |
| † Current address: Division of Systems Innovation, Graduate School of Engineering Science, Osaka University, Toyonaka 560-8531, Japan. E-mail: E-mail: horade@arai-lab.sys.es.osaka-u.ac.jp. |
| ‡ Current address: Department of Applied Chemistry, Graduate School of Engineering, FIRST Research Center for Innovative Nanobiodevices, Nagoya University, Nagoya, 464-8603, Japan. E-mail: E-mail: kaji@apchem.nagoya-u.ac.jp. |
| This journal is © The Royal Society of Chemistry 2013 |
