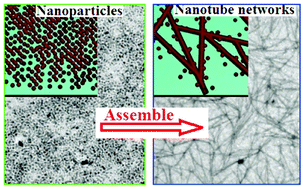
Beyond the superlattices, there are few other intricate superstructures that have been successfully assembled on a 2D substrate from nanocrystal building blocks. Here, we show the direct assembly of oleic acid-capped TiO2 nanocrystals into nanotube networks with good connectivity on 2D substrates. The assembly process was controlled by ethanol vapor. A combination of theoretical calculations and experimental data demonstrates a non-spontaneous assembly process with solvent vapour–ligand interactions, which is in agreement with the experimental results, but different from the widely reported nanocrystal superlattice assembly driven by entropy or enthalpy. From FT-IR spectra, we determined the occurrence of the solvent–ligand interaction; the oleic acid ligands of TiO2 nanocrystals transform into ethyl oleate due to the esterification of oleic acid by ethanol vapor, which may play a main role in driving the assembly process. Changing ethanol to tetrahydrofuran vapor prevented generation of nanotube-network structures, supplying indirect proof for this theory.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...