Enhanced performance in bulk heterojunction solar cells with alkylidene fluorene donor by introducing modified PFN-OH/Al bilayer cathode†
Soo Won Heo,
Kwan Wook Song and
Doo Kyung Moon*
Department of Materials Chemistry and Engineering, Konkuk University, 1 Hwayang-dong, Gwangjin-gu, Seoul, Republic of Korea. E-mail: dkmoon@konkuk.ac.kr; Fax: +82-2-444-0765
First published on 22nd November 2013
Abstract
We report bulk heterojunction solar cells with benzothiadiazole-containing polyalkylidene fluorene (PAFBToBT) donors made by introducing 2,4,7,9-tetramethyldec-5-yne-4,7-diol (Surfynol 104)-doped poly[9,9-bis(6′-(diethanolamino)hexyl)-fluorene] (PFN-OH) as an interfacial layer to enhance power conversion efficiency (PCE) and stability. Surfynol 104 has amphiphilic characteristics, and it supports the formation of a homogeneous interfacial layer on the photoactive layer. In addition, the application of the Surfynol 104-doped PFN-OH interfacial layer prevented the decrease in absorption of photons in the photoactive layer and improved the photocurrent density (JSC) by increasing the electron mobility. Therefore, JSC and the fill factor increased to 10.5 mA cm−2 and 49%, respectively, and the calculated PCE was 4.8%. In addition, a modified bilayer cathode system was introduced to the PCDTBT and the P3HT-based PSCs, and the PCE improved to 5.3% and 3.9%, respectively. Moreover, the in-air stability was enhanced.
1. Introduction
π-Conjugated polymer-based polymer solar cells (PSCs) offer the advantage of easy fabrication of light-weight devices with superior mechanical properties.1–3 Therefore, if spin-coating, ink jet printing, roll-to-roll printing, brush painting or stamping techniques are applied to the flexible substrate, light-weight and flexible, large-area photovoltaic devices can be fabricated inexpensively.4–9 However, the power conversion efficiency (PCE) of the PSCs at 8–9% remains too low for commercialization.10,11 Hence, studies have focused on improving PCE through the molecular structure design12,13 of a new donor polymer, the introduction of a bulk heterojunction (BHJ) system and the elucidation of BHJ device physics.14 To improve the PSC performance, research on device structure has recently focused on the optimization of photo-active layer formation and the introduction of an interfacial layer. For effective charge separation on the photo-active layer, a solvent with a different boiling point can be mixed during the manufacture of the polymer ink,15 or the carrier mobility can be enhanced by forming an interpenetrating polymer network after adding a processing additive.16–18 To improve the photon harvesting properties, a buffer layer with metal nanoparticles can be introduced,19,20 or a metal oxide layer can be inserted as an optical spacer.21 Other studies have improved the electron accepting properties through the introduction of a self-assembled monolayer22,23 in order to tune the work function of the indium tin oxide (ITO) anode, which has been used as a transparent electrode, or insert an interfacial layer such as LiF or BaF2 between the cathode and the photo-active layers.8,9,19,24 Because an interfacial layer such as LiF and BaF2 is introduced through thermal evaporation, some difficulties are encountered in manufacturing high-efficiency all-solution processed PSCs. In the Cao group, therefore, the short-circuit current density (JSC) and fill factor (FF) were reportedly improved by introducing an alcohol/water-soluble polymer by spin coating between the cathode and the photo-active layers.25 In theory, because an alcohol/water-soluble polymer is not intermixed with an active layer (organic solvent soluble polymer), a multi-layer structure can be fabricated through a solution process. According to a study by Zhang et al., even though the characteristics of the open circuit voltage (VOC), JSC and FF were improved because of the internal electric field induced by the use of poly(ethylene oxide) (PEO) as a cathode interfacial layer, the PCE was not significantly improved due to the insulating properties of PEO.26 The conjugated polymers for an interfacial layer with alcohol/water-soluble characteristics of good electron conductivity include poly[(9,9-dioctyl-2,7-fluorene)-alt-(9,9-bis(3′-(N,N-dimethylamino)propyl)-2,7-fluorene)] (PFN) and poly[9,9-bis(6′-(diethanolamino)hexyl)-fluorene] (PFN-OH).27,28 These two polymers dramatically improved the maximum brightness and power efficiency after being applied to polymer light-emitting diodes and white-polymer light-emitting diodes in advance.29 He et al. and Zhang et al. improved the PCE by 65% and 80%, respectively, by introducing PFN to carbazole-quinoxalinebased PSCs and benzotriazole-containing polycarbazole-based PSCs.25,30 Both PFN and PFN-OH showed good conductivity with a conjugated polyfluorene backbone structure. In addition, amino groups in the side chain acted to inject electrons that had been spun off from the photo-active layer into the cathode, while the surfactant-like side chain and –OH group exhibited good solubility in the alcohol-based solvent. However, it has been reported that they are ineffective in poly(3-hexylthiophene, P3HT)- and poly(ρ-phenylenevinylene, PPV)-based PSCs. Even though they are effective in the PSCs of the N-heterocycle-containing polymer donor system, a low PCE of 3% or less has been observed.25,30 In addition, even though the photo-active layer and alcohol/water-soluble polymer should not be intermixed with each other, the small molecules of an active layer have been removed by alcohol or water.29In this study, we confirm that the photoactive layer and the interlayer are not intermixed with each other after adding 2,4,7,9-tetramethyldec-5-yne-4,7-diol (Surfynol 104, AirProducts, USA) as a surfactant into the PFN-OH (alcohol/water-soluble polymer solution) as an interfacial layer material. After improving the photon harvesting properties by preventing a decrease in the absorbance and forming a good film morphology, the efficiency was enhanced by improving JSC and FF. In PSCs to which the poly[9-(1′-decylundecylidene)-2,7-fluorene-alt-5,5′-(4,7-bis(2-thienyl)-5,6-dioctyloxy-2,1,3-enzothiadiazole)] (PAFBToBT) was introduced, a 60% increase of PCE (3.0% → 4.8%) was observed. In addition, a 32% increase in PCE (4.0% → 5.3%) was detected in poly[N-9′-heptadecanyl-2,7-carbazolealt-5,5-(4′,7′-di-2-thienyl-2′,1′,3′-benzothiadiazole)](PCDTBT)31-based PSCs. Furthermore, in the P3HT-based PSCs, but not in the N-heterocycle-containing polymer donor system, PCE (3.9%) was improved by 30% when our developed system was introduced, unlike the previous studies in which no marked effect was observed even though PFN-OH was introduced as an interfacial layer.
2. Experiment
2.1. Materials
Poly(3,4-ethylenedioxythiophene) poly(styrene sulfonate) (PEDOT:PSS, AI 4083) as a hole transporting layer material was purchased from Clevios. P3HT, a donor material in the photo active layer, was purchased from Rieke Metals while the acceptor materials PC61BM and PC71BM were bought from Nano C. The Surfynol 104, which was used as an amphiphilic surfactant, was purchased from AirProducts. PAFBToBT (weight-average molecular weight (Mw): 37 kDa and Mw/Mn (Mn: number-average molecular weight): 2.07) was synthesized in accordance with the method in our previous study.31 PFN-OH (Mw: 15 kDa and Mw/Mn: 2.31) and PCDTBT (Mw: 35 kDa and Mw/Mn: 1.89) were synthesized in accordance with the method described in the literature.28,32 The chemical structures of Surfynol 104, PFN-OH, PAFBToBT, PCDTBT, P3HT, PC61BM and PC71BM are shown in Fig. 1. | ||
| Fig. 1 The chemical structures of (a) Surfynol 104, (b) PFN-OH, (c) PAFBToBT, (d) PCDTBT, (e) P3HT, (f) PC61BM and (g) PC71BM. | ||
2.2. Measurements
All of the thin films were fabricated using a GMC2 spin coater (Gensys), and their thicknesses were measured using an alpha step 500 surface profiler (KLA-Tencor). The morphology of the thin films were observed through atomic force microscopy (AFM, PSIA XE-100). The Raman spectroscopy measurements were performed using a T64000 (HORIBA Jobin Yvon). The current density–voltage (J–V) characteristics of the PSCs were measured using a Keithley 2400 source measure unit. The devices were evaluated at 298 K using a Class A Oriel solar simulator (Oriel 96000 150 W solar simulator) with a xenon lamp that simulates AM 1.5G irradiation (100 mW cm−2) from 400 to 1100 nm. The instrument was calibrated with a monocrystalline Si diode fitted with a KG5 filter to bring the spectral mismatch to unity. The calibration standard was calibrated by the National Renewable Energy Laboratory (NREL). The incident photon-to-current efficiency (IPCE) (Mc science) was measured. All devices were not encapsulated and stored in air for 600 hours in the dark for investigation of their long-term stability (Test ID: ISOS-D-1Shelf).332.3. Fabrication and characterization of the PSCs
To clean the ITO glass (10 Ω sq−1, Samsung Corning) and bare glass, they were sonicated in detergent (Alconox® in DI water, 10 wt%), acetone, isopropyl alcohol and DI water, in order, for 20 min and baked on a hot plate for 10 min at 100 °C. For the hydrophilic treatment of the ITO glass and bare glass surface, they were cleaned for 10 min in a UVO cleaner. On top of the ITO glass, the PEDOT:PSS was spin coated at 4000 rpm and a 40 nm-thick buffer layer was formed. Then, the solutions were annealed on the hot plate at 120 °C for 20 min to remove any residual solvent. In the case of an active layer, PAFBToBT and PCDTBT (donors) were mixed with PC71BM (acceptor) in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. In addition, P3HT and PC61BM were blended in the ratio of 1
4. In addition, P3HT and PC61BM were blended in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6. The solutions were blended with an ODCB concentration of 1.5 wt% and spin-coated on the buffer layer to form a 130 nm-thick layer and then annealed at 120–160 °C for 10 min. To form a bilayer cathode, the PFN-OH was dissolved in methanol + DI water (93
0.6. The solutions were blended with an ODCB concentration of 1.5 wt% and spin-coated on the buffer layer to form a 130 nm-thick layer and then annealed at 120–160 °C for 10 min. To form a bilayer cathode, the PFN-OH was dissolved in methanol + DI water (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v) + Surfynol 104 (0.1 wt%) at a concentration of 0.2 wt%. The solution was then spin coated onto the photo active layer to form a 5 nm-thick layer and then annealed at 80 °C for 10 min. To form the metal cathode, Al (5 Å s−1, 100 nm) was thermally deposited in a high-vacuum (<10−7 torr) chamber. Finally, PSCs with an active area of 4 mm2 were fabricated through encapsulation. For comparison, PSCs without a PFN-OH layer were also fabricated. The configuration of the cathode was BaF2 (0.1 Å s−1, 2 nm), Ba (0.2 Å s−1, 2 nm) and Al (5 Å s−1, 100 nm), which were thermally deposited in order in a high-vacuum chamber.
7 v/v) + Surfynol 104 (0.1 wt%) at a concentration of 0.2 wt%. The solution was then spin coated onto the photo active layer to form a 5 nm-thick layer and then annealed at 80 °C for 10 min. To form the metal cathode, Al (5 Å s−1, 100 nm) was thermally deposited in a high-vacuum (<10−7 torr) chamber. Finally, PSCs with an active area of 4 mm2 were fabricated through encapsulation. For comparison, PSCs without a PFN-OH layer were also fabricated. The configuration of the cathode was BaF2 (0.1 Å s−1, 2 nm), Ba (0.2 Å s−1, 2 nm) and Al (5 Å s−1, 100 nm), which were thermally deposited in order in a high-vacuum chamber.
3. Results and discussion
In terms of configuration, PSCs were structured with ITO (10 Ω sq−1, 170 nm)/PEDOT:PSS (40 nm)/active layer/cathode (Al (100 nm)) or (BaF2 (2 nm)/Ba (2 nm)/Al (100 nm)) and ITO/PEDOT:PSS/active layer/modified PFN-OH layer (5 nm)/Al (100 nm). The J–V characteristics and external quantum efficiency (EQE) of the PSCs which used PAFBToBT as an active layer and introduced various cathodes are shown in Fig. 2 and the results are summarized in Table 1. Moreover, the J–V characteristics and EQE of the PSCs using the PCDTBT and the P3HT as an electron donor polymer are shown in Fig. S1 and S2†. | ||
Fig. 2 (a) J–V characteristics and (b) external quantum efficiency (EQE) characteristics of PSCs with PAFBToBT:PC71BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 as the active layer when using various cathodes. 4 as the active layer when using various cathodes. | ||
| Polymer | Cathode | Voc [V] | Jsc [mA cm−2] | FF [%] | PCE [%] | Rs [Ω cm2] |
|---|---|---|---|---|---|---|
PANBToBT:PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
Al (ref.) | 0.878 | 8.9 | 38.7 | 3.0 | 53.7 |
| BaF2/Ba/Al | 0.878 | 8.9 | 45.7 | 3.6 | 35.8 | |
| PFN-OH + MeOH/Al | 0.930 | 9.3 | 47.5 | 4.1 | 21.3 | |
| PFN-OH + MeOH + DI water/Al | 0.939 | 9.6 | 47.1 | 4.2 | 18.5 | |
| PFN-OH + DI water + Surfynol 104/Al | 0.939 | 10.5 | 49 | 4.8 | 16.9 | |
PCDTBT:PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
Al | 0.858 | 9.3 | 49.5 | 4.0 | 42.5 |
| PFN-OH + DI water + Surfynol 104/Al | 0.959 | 9.7 | 58.5 | 5.3 | 16.3 | |
P3HT:PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6) 0.6) |
Al | 0.616 | 7.7 | 62.6 | 3.0 | 17.2 |
| PFN-OH + DI water + Surfynol 104/Al | 0.621 | 9.2 | 68.6 | 3.9 | 12.6 |
The VOC, JSC and FF values of the PSCs which used only Al as the cathode were 0.878 V, 8.9 mA cm−2 and 38.7%, respectively, and the calculated PCE was 3.0%. However, when Surfynol 104 was added to PFN-OH at 0.1 wt%, the device performance was optimized and the calculated PCE was 4.8% (the optimization data of devices are shown in ESI Fig. S3†). This result was improved by about 60% compared to the reference device which was the applied Al anode. The important thing is that JSC and FF significantly increased after adding Surfynol 104 to PFN-OH. Therefore, it is expected that the characteristics of the interfacial layer changed with the addition of Surfynol 104 to PFN-OH. For analysis of this change, various surface analyses were conducted.
As shown in Fig. 3, the root mean square (RMS) roughness of the surface morphology increased from 0.491 nm when PFN-OH was dissolved in methanol to 0.734 nm when PFN-OH was dissolved in the methanol and DI water-mixed solution because of the formation of larger agglomerate domains of PFN-OH molecules in the interfacial layer. The increased agglomerate domain size of the PFN-OH molecules improved the mobility in the agglomerate domain because PFN-OH has an amino group in the chain, and the mobility increased in the chain at the domain formation. However, the looseness of these agglomerate domains prevented their conductivity from increasing (Fig. 4(a)).34 Hence, despite the increase in JSC, FF slightly decreased because of the increased surface roughness after the formation of the agglomerate domain. As a result, PCE slightly increased from 4.1 to 4.2%.
 | ||
| Fig. 3 AFM images of (a) PFN-OH + MeOH, (b) PFN-OH + DI water, (c) PFN-OH + MeOH + DI water and (d) PFN-OH + MeOH + DI water + Surfynol 104. | ||
Fig. 4 shows the mechanism of forming a lower surface roughness film of PFN-OH with Surfynol 104 compared to the pristine PFN-OH film. Surfynol 104 is an amphiphilic surfactant which consists of a hydrophobic alkyl backbone and hydrophilic hydroxyl group. Hence, hydrophobic/hydrophobic interactions such as van der Waals interactions and other multiple weak interactions take place in the hydrophobic backbone of the Surfynol 104 in PFN-OH and the hydrophobic photoactive layer surface.23 Therefore, the surface of the photoactive layer became hydrophilic because of the hydroxyl group in Surfynol 104. As a result, hydrophilic PFN-OH can form a lower surface roughness film on the photoactive layer. Therefore, because a tight agglomerate domain (RMS: 0.564 nm) can be formed by creating a domain which is bigger than methanol but finer than the methanol and DI water mixed solution at the formation of the agglomerate domain of PFN-OH, the electrons that were generated in the photo active layer can be quickly extracted through the external circuit by increasing the conductivity of the PFN-OH thin film (Fig. 4(b)).
Charge mobility studies of the devices with a modified PFN-OH interfacial layer as the electron collection layer were conducted with electron-only devices using space charge limited current (SCLC) methods.35 Electron-only devices were fabricated with a diode configuration of ITO/Cs2CO3/PAFBToBT:PC71BM/with or without modified PFN-OH/Al. The graphs of the logarithm of JL3/V2 versus the square root of the mean electric field are shown in Fig. 5. The electron mobility of the devices with PCDTBT and P3HT are shown in Fig. S4.† The electron mobility of the pristine device was calculated to be 1.66 × 10−3 cm2 V−1 s−1, whereas the device with a modified PFN-OH layer had a value as high as 3.73 × 10−3 cm2 V−1 s−1. The electron mobility was about 2-fold higher than that of the pristine device. Because of the high electron mobility of the device with modified PFN-OH, therefore, the RS decreased from 53.7 Ω cm2 to 16.9 Ω cm2.
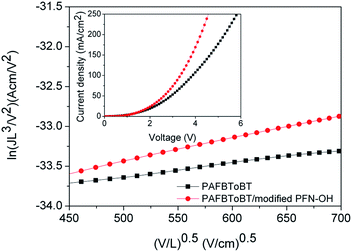 | ||
| Fig. 5 The logarithm of JL3/V2 versus the square root of the mean electric field of electron-only devices measured in the dark. (Inset: J–V characteristics of electron-only devices). | ||
To study the structural properties of the Surfynol 104 doped PFN-OH layer on the photoactive layer, Raman spectra were measured for the pristine PFN-OH + MeOH/PAFBToBT, and the Surfynol 104 doped PFN-OH + MeOH + DI water/PAFBToBT, as shown in Fig. 6. The spectrum of the Surfynol 104 doped PFN-OH + MeOH + DI water/PAFBToBT was very similar to the corresponding spectrum of the pristine PFN-OH + MeOH/PAFBToBT. From Raman spectroscopy it was revealed that the Surfynol 104 was removed from the PFN-OH layer when PFN-OH was annealed at 80 °C. Therefore, we confirmed that the electron mobility of the interfacial layer does not change due to the Surfynol 104 in the presence of the PFN-OH film.
 | ||
| Fig. 6 Raman spectra of PFN-OH + MeOH/PAFBToBT, Surfynol 104 doped PFN-OH + MeOH + DI water/PAFBToBT and Surfynol 104. | ||
Fig. 7 shows the UV-absorption spectra of the PAFBToBT film treated with methanol, methanol + DI water and methanol + DI water + Surfynol 104. According to Fig. 7, almost no change was observed in the absorbance of the PAFBToBT film washed with the methanol, DI water and Surfynol 104-mixed solution. Therefore, PFN-OH was treated with the Surfynol 104 solution introducing an interfacial layer, without decreasing the photon harvesting properties.
Fig. 8 shows the result of the air stability test on the PSCs which introduced the Al cathode and Surfynol 104-doped PFN-OH/Al bilayer cathode, which was performed for 600 h. In the modified PFN-OH based devices, 37% degradation was observed after 600 hours. In the reference PSC, however, PCE decreased up to about 1/5 of the beginning value during the same period. It has been confirmed that the long-term stability was increased through the introduction of the modified PFN-OH to the cathode electrode.
 | ||
| Fig. 8 PCE as a function of storage time for PAFBToBT based PSCs fabricated with an Al cathode and the Surfynol 104-doped PFN-OH/Al bilayer cathode in air under ambient conditions (no encapsulation). | ||
4. Conclusion
PAFBToBT-based PSCs have been fabricated by introducing surfactant-doped PFN-OH for enhancing the PCE and the stability. Surfynol 104 has amphiphilic characteristics, and it supported the formation of a homogeneous interfacial layer on the photoactive layer. In addition, the application of the Surfynol 104-added PFN-OH interfacial layer prevented the decrease in absorption of photons in the photoactive layer and improved JSC by increasing the electron mobility. Therefore, JSC and FF were increased to 10.5 mA cm−2 and 49%, respectively, and the calculated PCE was 4.8%. In addition, when a modified bilayer cathode system was introduced to the PCDTBT and the P3HT-based PSCs, the PCE was improved to 5.3% and 3.9%, respectively. Moreover, the in-air stability was enhanced.Acknowledgements
This research was supported by a grant (10037195) from the Fundamental R&D Program for Core Technology of Materials funded by the Ministry of Knowledge Economy, Republic of Korea and the National Research Foundation of Korea Grant funded by the Korean Government (MEST) (NRF-2009-C1AAA001-2009-0093526).References
- H.-Y. Chen, J. H. Hou, S. Q. Zhang, Y. Y. Liang, G. W. Yang, Y. Yang, L. P. Yu, Y. Wu and G. Li, Nat. Photonics, 2009, 3, 649 CrossRef CAS.
- Y. Liang, Z. Xu, J. Xia, S.-T. Tsai, Y. Wu, G. Li, C. Ray and L. Yu, Adv. Mater., 2010, 22, E135 CrossRef CAS PubMed.
- J. Y. Lee, W. S. Shin, J. R. Haw and D. K. Moon, J. Mater. Chem., 2009, 19, 4938 RSC.
- F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2009, 93, 394 CrossRef CAS PubMed.
- E. Bundgaard, O. Hagemann, M. Manceau and M. Jørgensen, Macromolecules, 2010, 43, 8115 CrossRef CAS.
- M. Manceau, D. Angmo, M. Jørgensen and F. C. Krebs, Org. Electron., 2011, 12, 566 CrossRef CAS PubMed.
- F. C. Krebs, J. Fyenbo and M. Jørgensen, J. Mater. Chem., 2010, 20, 8994 RSC.
- S. W. Heo, J. Y. Lee, H. J. Song, J. R. Ku and D. K. Moon, Sol. Energy Mater. Sol. Cells, 2011, 95, 3041 CrossRef CAS PubMed.
- S. W. Heo, K. W. Song, M. H. Choi, T. H. Sung and D. K. Moon, Sol. Energy Mater. Sol. Cells, 2011, 95, 3564 CrossRef CAS PubMed.
- Z. He, C. Zhong, X. Huang, W.-Y. Wong, H. Wu, L. Chen, S. Su and Y. Cao, Adv. Mater., 2011, 23, 4636 CrossRef CAS PubMed.
- Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591 Search PubMed.
- D. Kitazawa, N. Watanabe, S. Yamamoto and J. Tsukamoto, Appl. Phys. Lett., 2009, 95, 053701 CrossRef PubMed.
- E. Wang, L. Hou, Z. Wang, S. Hellstrom, F. Zhang, O. Inganas and M. R. Andersson, Adv. Mater., 2010, 22, 5240 CrossRef CAS PubMed.
- P. W. M. Blom, V. D. Mihailetchi, L. J. A. Koster and D. E. Markov, Adv. Mater., 2007, 19, 1551 CrossRef CAS.
- F. Zhang, K. G. Jespersen, C. Bjorstrom, M. Svensson, M. R. Andersson, V. Sundstrom, K. Magnusson, E. Moons, A. Yartsev and O. Inganas, Adv. Funct. Mater., 2006, 16, 667 CrossRef CAS.
- J. K. Lee, W. L. Ma, C. J. Brabec, J. Yuen, J. S. Moon, J. Y. Kim, K. Lee, G. C. Bazan and A. J. Heeger, J. Am. Chem. Soc., 2008, 130, 3619 CrossRef CAS PubMed.
- S. W. Heo, S. H. Kim, E. J. Lee and D. K. Moon, Sol. Energy Mater. Sol. Cells, 2013, 111, 16 CrossRef CAS PubMed.
- S. W. Heo, K. W. Song, M. H. Choi, H. S. Oh and D. K. Moon, Sol. Energy Mater. Sol. Cells, 2013, 114, 82 CrossRef CAS PubMed.
- S. W. Heo, I. S. Song, Y. S. Kim and D. K. Moon, Sol. Energy Mater. Sol. Cells, 2012, 101, 295 CrossRef CAS PubMed.
- S. W. Heo, E. J. Lee, K. W. Song, J. Y. Lee and D. K. Moon, Org. Electron., 2013, 14, 1931 CrossRef CAS PubMed.
- A. Roy, S. H. Park, S. Cowan, M. H. Tong, S. Cho, K. Lee and A. J. Heeger, Appl. Phys. Lett., 2009, 95, 013302 CrossRef PubMed.
- M. G. Helander, Z. B. Wang, J. Qiu, M. T. Greiner, D. P. Puzzo, Z. W. Liu and Z. H. Lu, Science, 2011, 332, 944 CrossRef CAS PubMed.
- S. W. Heo, K. H. Baek, T. H. Lee, J. Y. Lee and D. K. Moon, Org. Electron., 2013, 14, 1629 CrossRef CAS PubMed.
- C. J. Brabec, S. E. Shaheen, C. Winder, N. S. Sariciftci and P. Denk, Appl. Phys. Lett., 2002, 80, 1288 CrossRef CAS PubMed.
- L. Zhang, C. He, J. Chen, P. Yuan, L. Huang, C. Zhang, W. Cai, Z. Liu and Y. Cao, Macromolecules, 2010, 43, 9771 CrossRef CAS.
- F. Zhang, M. Ceder and O. Inganas, Adv. Mater., 2007, 19, 1835 CrossRef CAS.
- H. Wu, F. Huang, Y. Mo, W. Yang, D. Wang, J. Peng and Y. Cao, Adv. Mater., 2004, 16, 1826 CrossRef CAS.
- F. Huang, Y. H. Niu, Y. Zhang, J. W. Ka, M. S. Liu and A. K. Y. Jen, Adv. Mater., 2007, 19, 2010 CrossRef CAS.
- D. An, J. Zou, H. Wu, J. Peng, W. Yang and Y. Cao, Org. Electron., 2009, 10, 299 CrossRef CAS PubMed.
- Z. He, C. Zhang, X. Xu, L. Zhang, L. Huang, J. Chen, H. Wu and Y. Cao, Adv. Mater., 2011, 23, 3086 CrossRef CAS PubMed.
- K. W. Song, H. J. Song, T. H. Lee, S. W. Heo and D. K. Moon, Polym. Chem., 2013, 4, 3225 RSC.
- N. Blouin, A. Michaud and M. Leclerc, Adv. Mater., 2007, 19, 2295 CrossRef CAS.
- M. O. Reese, S. A. Gevorgyan, M. Jørgensen, E. Bundgaard, S. R. Kurtz, D. S. Ginley, D. C. Olson, M. T. Lloyd, P. Morvillo, E. A. Katz, A. Elschner, O. Haillant, T. R. Currier, V. Shrotriya, M. Hermenau, M. Riede, K. R. Kirov, G. Trimmel, T. Rath, O. Inganäs, F. Zhang, M. Andersson, K. Tvingstedt, M. Lira-Cantu, D. Laird, C. McGuiness, S. Gowrisanker, M. Pannone, M. Xiao, J. Hauch, R. Steim, D. M. DeLongchamp, R. Rösch, H. Hoppe, N. Espinosa, A. Urbina, G. Yaman-Uzunoglu, J.-B. Bonekamp, A. J. J. M. van Breemen, C. Girotto, E. Voroshazi and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2011, 95, 1253 CrossRef CAS PubMed.
- Y. Zhang, F. Huang, Y. Chi and A. K.-Y. Jen, Adv. Mater., 2008, 20, 1565 CrossRef CAS.
- S. Jeong, Y. Kwon, B. D. Choi, H. Ade and Y. S. Han, Appl. Phys. Lett., 2010, 96, 183305 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra42263a |
| This journal is © The Royal Society of Chemistry 2014 |


