Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chlorophyll tailored 20-trifluoroacetamide and its azacrown derivative as pH sensitive colorimetric sensor probe with response to AcO−, F− and CN− ions†
Vladimir
Iashin
a,
Tatyana V.
Koso
a,
Kati
Stranius
b,
Mikko
Muuronen
a,
Sami
Heikkinen
a,
Jari
Kavakka
a,
Nikolai V.
Tkachenko
b and
Juho
Helaja
*a
aLaboratory of Organic Chemistry, Department of Chemistry, University of Helsinki, A.I. Virtasen aukio 1, P.O. Box 55, FIN-00014, Finland. E-mail: juho.helaja@helsinki.fi; Fax: +358919150466; Tel: +358919150369
bInstitute of Materials Chemistry, Tampere University of Technology, P. O. Box 541, 33101 Tampere, Finland. E-mail: nikolai.tkachenko@tut.fi
First published on 17th May 2013
Abstract
Chlorophyll derivatives were functionalized with a trifluoroamide (TFA) group to obtain colorimetric ion sensors. Prior to this, a NO2BF4*pyridine based, chlorin nitration method was developed. The base strength dependent sensing of AcO−, F− and CN− ions was detected in aqueous media, which was enhanced in nonprotic solvents by an integration of the ditopic chelation capable amine crown-6 moiety.
Despite chlorophylls (Chls) known excellence in photosynthetic light absorption and excitation processes1 there are only very few examples of Chl derived sensor molecules in the literature.2 Nevertheless, Tamiaki and co-workers have shown convincingly that chlorins functionalized with a trifluoroacetyl group at the 3-position show ratiometric colorimetric sensing abilities towards amines.2 To study whether chlorins could act as a chromophoric platform beyond this example we decided to integrate a trifluoroacetamide (TFA) group at the chlorin meso position to develop colorimetric F− and CN− ion sensors. Previously, TFA has been integrated as a sensor probe in several chromophores to show selective “naked eye” detection properties in aqueous media.3 Generally, selective, simple and inexpensive optical sensing, especially for the CN− ion,4 would be exceedingly desirable due to its detrimental environmental and health effects as well as its importance in process chemistry and mining.5
In advance to chlorin amide functionalization, we developed a selective nitration method for the chlorin ring. It is well known that the 20-position is prone to electrophilic aromatic substitution reactions e.g. halogenations, cyanation and formylation.6 Consistently, Smith and co-workers developed the pioneering selective nitration method of mesopyro-pheophorbide a (1a) at the 20-position that was based on the use of thallium(III) nitrate as the nitrate source yielding 32% after several synthetic operations and flash purification.6c In order to develop the environmentally benign large scale nitration of chlorin, we investigated the feasibility of a transfer nitration of a chlorin ring. To apply this methodology, we piloted the use of NO2BF4 as the chlorin ring nitration reagent. In our initial attempts we noticed that the NO2BF4 in its pure form was excessively hygroscopic for operations under atmosphere and also a far too vigorous reagent for selective nitration. Consequently, we set out to explore the nitration with NO2BF4 in the presence of small amounts of water in DCM at room temperature. By this approach the nitration became to some extent successful, but still with inconsistent yields. The technical challenge was the poor reproducibility of the reaction; the yield of the reaction was very sensitive to the ambient amount of moisture. However, Olah et al. have reported that the nitration of aromatic rings could be carried out with heterocyclic base complexes of NO2BF4 in nearly neutral reaction conditions.7 These reagents are slightly less reactive but far more user-friendly. The complexes can be easily prepared by the addition of an equimolar amount of a selected heterocyclic base to NO2BF4 dissolved in acetonitrile as described by Olah et al.7 The NO2BF4 complexes are no longer amorphous hygroscopic, but a crystalline material, which can be stored for longer periods of time. When chlorin 1a was treated with N-nitropyridinium tetrafluoroborate in DCM at room temperature, very good yields were obtained with perfect repeatability. Pleasingly, the nitration method turned out to be chemoselective and sufficiently soft so that even more chemically delicate chlorins, methyl esters of pyropheophorbide a (1b) and pheophorbide a (1c), were nitrated with equally high yields.
After having straightforward access to the 20-nitro chlorin 2a, the compound was converted to trifluoroamide 3 by reducing the nitro group to amine with Pt/C catalysis with acid additive under a hydrogen atmosphere in THF–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1),8 removing the solvents under vacuum and treating the mixture with trifluoroacetic anhydride (TFAA) in DCM in the presence of a catalytic amount of pyridine. Furthermore, to explore whether the aimed anion addition to the TFA carbonyl could be solidified by adjacent counter cation chelation, we integrated an aza-18-crown[6] ether moiety to form a propionic amide side chain. The methyl ester of chlorin 3 was hydrolysed to acid in 30% HCl after which the amide coupling was carried out with the aid of EDCI.
1),8 removing the solvents under vacuum and treating the mixture with trifluoroacetic anhydride (TFAA) in DCM in the presence of a catalytic amount of pyridine. Furthermore, to explore whether the aimed anion addition to the TFA carbonyl could be solidified by adjacent counter cation chelation, we integrated an aza-18-crown[6] ether moiety to form a propionic amide side chain. The methyl ester of chlorin 3 was hydrolysed to acid in 30% HCl after which the amide coupling was carried out with the aid of EDCI.
Our preliminary mechanistic scenario of the anion sensing was that the nucleophilic salts would react via nucleophilic 1,2-addition on the carbonyl of chlorin integrated TFA 3 → 3′ (Fig. 1). Alternatively, poorer nucleophiles could act as base and deprotonate TFA NH3 → 3′′. In both cases polarization would be induced on the chlorin macrocycle which is expected to cause a colour change. Because the ionic forms 3′ or 3′′ are formed in a reversible manner and the product is thermodynamically rather unstable we introduced the crown moiety to shift this equilibrium on the ionic side.
 | ||
| Fig. 1 Possible anion sensing mechanisms. | ||
 | ||
| Scheme 2 Derivation of 20-nitro-chlorin 2a to sensor molecules 3 and 4. | ||
We initiated the titration experiments with compound 3 using UV-vis spectroscopy monitoring in chloroform by adding tert-butylammonium fluoride (TBAF) in equivalents (Fig. 2). Encouragingly, the salt addition induced clear spectral changes, which were most pronounced for the Soret band (410 → 432 nm). Disappointingly, the sensitivity was rather modest, while an extent of 15 equivalents of F− salt was required to reach complete spectral changes. Instead, when the titration was carried out in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (95
H2O (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), a large excess of TBAF (150 equiv.) was added without notable effect. However, similar changes as above took place in a reversible manner when the temperature of this mixture was gradually varied in a range from 20 to 80 °C (Fig. S1, ESI†). Obviously the aqueous environment solvates (hydrates) the F− ions more efficiently and renders them from the interaction.9 When corresponding titrations were performed for compound 3 with CN− salts very similar spectral changes were observed.†
5), a large excess of TBAF (150 equiv.) was added without notable effect. However, similar changes as above took place in a reversible manner when the temperature of this mixture was gradually varied in a range from 20 to 80 °C (Fig. S1, ESI†). Obviously the aqueous environment solvates (hydrates) the F− ions more efficiently and renders them from the interaction.9 When corresponding titrations were performed for compound 3 with CN− salts very similar spectral changes were observed.†
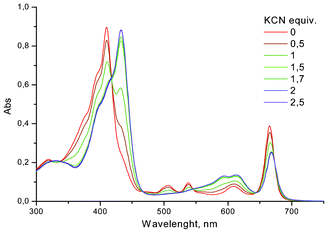
Next, we carried out the titration of compound 4 in an analogous manner with KF and KCN salts. Potassium was chosen as a cation due to its known affinity towards the crown[6]. Pleasingly, a complete conversion was reached in DMF with 1% water for both of the salts after addition of two equivalents of salts (Fig. 3). The presence of the crown improved the sensitivity almost by an order of magnitude. Yet, the limitation in compound 4 was a lack of selectivity between the F− and CN− anions in the studied solutions. The sensitivity and selectivity was also similar in DMSO (Fig. S2, ESI†). In this perspective the poor response measured for F− in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O inspired us to study the role of the added water.
H2O inspired us to study the role of the added water.
In the graph depicted in Fig. 4a the DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio is varied on the x-axis, while the values on the y-axis denote the KF and KCN equivalents to achieve complete conversion of 4 to 4′/4′′. The graph reveals that in the absence of water the compound has no selectivity for the ions, but at 95
water ratio is varied on the x-axis, while the values on the y-axis denote the KF and KCN equivalents to achieve complete conversion of 4 to 4′/4′′. The graph reveals that in the absence of water the compound has no selectivity for the ions, but at 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v the DMF–water ratio points start to follow a different trend. Distinctly, with 6% water content (DMF
5 v/v the DMF–water ratio points start to follow a different trend. Distinctly, with 6% water content (DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, 94
H2O, 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 15 equiv. of KF are needed for the full conversion, while the same result is obtained with less than five equivalents of KCN. This means that by adjusting the water content selective sensing for the CN− ion can be obtained. The titration with various selected potassium ions illustrated in Fig. 4b reveals that with 2.5 equiv. ion loading in DMF with 6% water the CN− ion is somewhat discriminated. However, by increasing the water content to 15 and 30% the discrimination seemed to become quantitative. It is noteworthy that the OH− ion gave an intense response in each case, which indicates that basic conditions are not suitable for selective ion sensing. Disappointingly, adjusting the pH value to 9.2 with carbonate buffer, i.e. at the pKa level of HCN, gave a colour change. Meanwhile, regulating pH at neutral level in aqueous media revealed that the sensing was drastically damped down.† These experiments implied that in dry conditions the observed colorimetric changes are caused by AcO−, F− and CN− anions by the base strengths,10 while in aqueous media the OH− anions produced by the salt hydrolysis have a probable competitive role.
6) 15 equiv. of KF are needed for the full conversion, while the same result is obtained with less than five equivalents of KCN. This means that by adjusting the water content selective sensing for the CN− ion can be obtained. The titration with various selected potassium ions illustrated in Fig. 4b reveals that with 2.5 equiv. ion loading in DMF with 6% water the CN− ion is somewhat discriminated. However, by increasing the water content to 15 and 30% the discrimination seemed to become quantitative. It is noteworthy that the OH− ion gave an intense response in each case, which indicates that basic conditions are not suitable for selective ion sensing. Disappointingly, adjusting the pH value to 9.2 with carbonate buffer, i.e. at the pKa level of HCN, gave a colour change. Meanwhile, regulating pH at neutral level in aqueous media revealed that the sensing was drastically damped down.† These experiments implied that in dry conditions the observed colorimetric changes are caused by AcO−, F− and CN− anions by the base strengths,10 while in aqueous media the OH− anions produced by the salt hydrolysis have a probable competitive role.
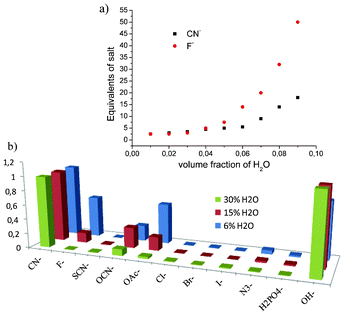 | ||
Fig. 4 a) The required salt equivalents to reach complete (>99%) conversion of sensor 4 to 4′/4′′ (8.6 × 10−6 M) in variable DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O v/v mixtures. b) Increase in UV-vis absorbance at 432 nm in the presence of 2.5, 25 and 40 equiv. ions with 6, 15 and 30% water content in DMF, respectively. H2O v/v mixtures. b) Increase in UV-vis absorbance at 432 nm in the presence of 2.5, 25 and 40 equiv. ions with 6, 15 and 30% water content in DMF, respectively. | ||
To get some theoretical insight in to the sensing mechanism a computational study was undertaken at the DFT level in solution (TPSS-D3 and PBE-D3/def2-TZVP).† In a contrast to the recent report on TFA-based cyanide sensor probes,3g the computed energetics of our cation included systems favoured amide deprotonation clearly (4 → 4′′) over the addition (4 → 4′) mechanism for both the F− and the CN− ions by 11.4 and 14.3 kcal mol−1 (TPSS-D3), respectively (Fig. 5, Fig. S3 and S4, ESI†). Experimentally, the measured 19F-13C HMBC and 19F-19F COSY NMR data failed to give any evidence for covalent bonding that would indicate nucleophilic addition of CN− and F− anions.† Also, the small (ΔδC ≈ 1 ppm) shielding value of the TFA carbonyl carbon in the presence of TBACN (and TBAF) suggests the deprotonation rather than the addition mechanism.†
 | ||
| Fig. 5 TPSS-D3/def2-TZVP optimized geometries: on the left, KCN-4′, carbonyl carbon addition and on right, KCN-4′′, amide deprotonated KCN complexes of 4. Deprotonation is favoured by 14.3 kcal mol−1 over addition. | ||
In conclusion, we have developed a soft, regioselective, NO2BF4 pyridine complex based transfer nitration method for chlorin compounds. Subsequently, the nitro group was converted to TFA functionality by reduction and acylation. This compound showed similar colorimetric anion sensing properties for nucleophilic AcO−, F− and CN− anions in dry nonprotic solvents. The suggested sensing mechanism takes place via deprotonation of the TFA amide proton. Anion sensitivity but not selectivity could be improved by introducing a crown moiety for the chelation of the counter cations. We anticipate that this result is not sensor probe specific, but rather general for probes based on related mechanisms. Therefore, pertinent solvent systems should be systematically studied when sensor properties are manifested.
This work was partially supported by the Academy of Finland [No. 135113 and 135058]. Dr Petri Heinonen is accredited for the HRMS measurements. The National Centre for Scientific Computing (CSC) is acknowledged for computational resources.
Notes and references
- H. Scheer, Advances in Photosynthesis and Respiration, in Chlorophylls and Bacteriochlorophylls, ed. B. Grimm, R. J. Porra, W. Rüdiger and H. Scheer, Springer, Dordrecht, Netherlands, 2006, 25, pp. 1–26 Search PubMed.
- (a) S.-i. Sasaki, Y. Kotegawa and H. Tamiaki, Tetrahedron Lett., 2006, 47, 4849–4852 CrossRef CAS; (b) S.-i. Sasaki, Y. Kotegawa and H. Tamiaki, Bull. Chem. Soc. Jpn., 2009, 82, 267–271 CrossRef CAS.
- (a) Y. M. Chung, B. Raman, D.-S. Kim and K. H. Ahn, Chem Commun., 2006, 186–188 RSC; (b) H.-T. Niu, X. Jiang, J. He and J.-P. Cheng, Tetrahedron Lett., 2008, 49, 6521–6524 CrossRef CAS; (c) H.-T. Niu, D. Su, X. Jiang, W. Yang, Z. Yin, J. He and J.-P. Cheng, Org. Biomol. Chem., 2008, 6, 3038–3040 RSC; (d) H. Yu, Q. Zhao, Z. Jiang, J. Qin and Z. Li, Sensors and Actuators B, 2010, 148, 110–116 CrossRef CAS; (e) C.-O. Ng, S.-W. Lai, H. Feng, S.-M. Yiu and C.-C. Ko, Dalton Trans., 2011, 40, 10020–10028 RSC; (f) J. Isaad and A. El Achari, Tetrahedron, 2011, 67, 4196–4201 CrossRef CAS; (g) H. Li, B. Li, L.-Y. Jin, Y. Kan and B. Yin, Tetrahedron, 2011, 67, 7348–7353 CrossRef CAS.
- Z. Xu, X. Chen, H. N. Kim and J. Yoon, Chem. Soc. Rev., 2010, 39, 127–137 RSC.
- C. Young, L. Tidwell and C. Anderson, Cyanide: Social, Industrial, and Economic Aspects, Minerals, Metals, and Materials Society, Warrendale, 2001 Search PubMed.
- (a) R. B. Woodward and V. Škarić, J. Am. Chem. Soc., 1961, 83, 4676–4678 CrossRef CAS; (b) P. H. Gore, F. S. Kamounah and A. Y. Miri, Tetrahedron, 1979, 35, 2927–2929 CrossRef CAS; (c) K. M. Smith, D. A. Goff and D. J. Simpson, J. Am. Chem. Soc., 1985, 107, 4946–4954 CrossRef CAS; (d) T. J. Michalski, E. H. Appelmann, M. K. Bowman, J. E. Hunt, J. R. Norris, T. M. Cotton and L. Raser, Tetrahedron Lett., 1990, 31, 6847–6850 CrossRef CAS.
- (a) G. A. Olah, J. A. Olah and N. A. Overchuk, J. Org. Chem., 1965, 30, 3373–3376 CrossRef CAS; (b) C. A. Cupas and R. L. Pearson, J. Am. Chem. Soc., 1968, 90, 4742–4743 CrossRef CAS.
- Catalytic hydrogenation trials of 2 with Pd/C and also with Pt/C without acid additive produced blue uncharacterized product.
- A. Bianchi and E. García-Espana, in Anion Coordination Chemistry, ed. K. Bowman-James, A. Bianchi and E. García-Espana, Wiley-WCH, Weinheim, Germany, 2012, pp. 75–140 Search PubMed.
- F. Boardwell, Acc. Chem. Res., 1988, 21, 456–463 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: synthetic procedures, compound characterizations, UV-vis titrations and computational chemistry. See DOI: 10.1039/c3ra41741g |
| This journal is © The Royal Society of Chemistry 2013 |