Self-healing polymers
Howard Colquhouna and Bert Klumpermanb
aDepartment of Chemistry, University of Reading, Whiteknights, Reading, RG6 6AD, UK
bDepartment of Chemistry and Polymer Science, Stellenbosch University, 7602 Matieland, South Africa
 Howard Colquhoun | Howard Colquhoun |
 Bert Klumperman | Bert Klumperman |
Polymeric materials are crucial to virtually all modern technologies from microelectronics to medicine, as exemplified by photoresists, injection-moulded engineering components, textile and technical fibres, surface-coatings, lightweight aerospace composites, prosthetic limbs, dental fillings, drug-delivery systems and replacement heart valves. However, the continuous exposure of polymers to abrasion, impact, and mechanical, chemical or thermal stress can lead to degradation of their physical properties and so, potentially, to the mechanical failure of the material. Polymers which can repair themselves when damaged should show significantly enhanced durability and lifetime in applications, and the development of such materials is currently a topic of intense investigation around the world. A wide range of new research on self-healing polymers is highlighted in this themed issue of Polymer Chemistry, which includes contributions from many of the leading investigators in the field.
The problem of achieving healability in polymer systems has been approached from many different directions. Ideally the damage should itself trigger a healing response, and materials where this occurs (generally referred to as autonomously healable systems) include polymers containing embedded microcapsules of liquid monomers that are released upon the material being damaged, which subsequently polymerise to heal the fracture. Recent developments in this area are highlighted in the mini-review by Shchukin (DOI: 10.1039/c3py00082f). A dramatic mode of autonomous healing is shown by certain ionomers which, especially in the acid form, achieve almost instant self-repair on being punctured by a high-velocity projectile, i.e. a bullet will pass through a sheet of polymer without leaving a hole! Here Kalista and co-workers report on the relationship between ionomer composition and this type of healing response (Fig. 1) which, although clearly driven by the thermal energy of impact, is highly material-specific and so does not, evidently, result from a classical chain-reptation mechanism (DOI: 10.1039/c3py00095h).
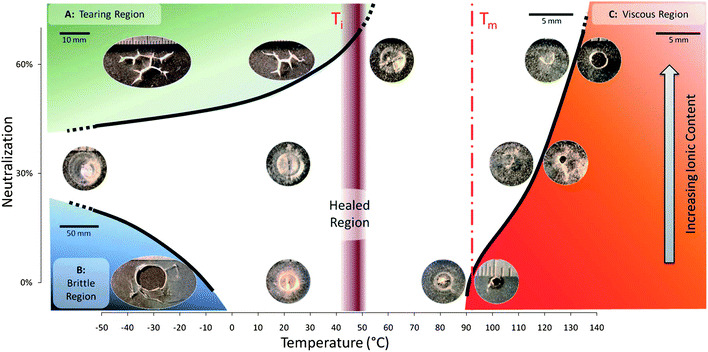 | ||
| Fig. 1 Self-healing “phase diagram” showing ballistic puncture-reversal as a function of temperature and ionic content (from the article by Kalista, Pflug and Varley, DOI: 10.1039/c3py00095h). | ||
Groote, Jakobs and Sijbesma review a rather subtle approach to the development of self-healing polymers that involves mechanochemical activation of a latent catalyst embedded in a polymer chain (DOI: 10.1039/c3py00071k). High-MW polymer chains are susceptible to mechanically induced chain-scission, and if the chain is designed so that scission activates a catalytic site, then in principle mechanical damage can again provide a trigger for initiating a specific self-healing reaction such as alkene metathesis, transesterification, or the anionic polymerisation of acrylate-type monomers.
Supramolecular approaches to healable polymer systems are the subject of a mini-review by Hart et al. (DOI: 10.1039/c3py00081h). Here the premise is that polymer chains of low molecular weight can be linked together by multiple non-covalent interactions such as hydrogen bonding, π–π-stacking or metal–ligand bonding, to give materials with mechanical properties more typical of high-MW polymers. Healability in such systems often requires a stimulus such as heat or light to disengage the supramolecular interactions and so enable the material to flow freely, but Guan and coworkers show, in this themed issue, that genuinely autonomous healing can be achieved in hydrogen-bonded nanoparticle systems (DOI: 10.1039/c3py00078h). Here, a polystyrene core is grafted with amide-functionalised polyacrylate brushes to give a “hairy” nanoparticle that self-assembles into a stiff, cohesive material through inter-particle hydrogen-bonding between the amide units (Fig. 2). Fractures in such systems heal autonomously at ambient temperatures through dissociation and re-association of the low-Tg, hydrogen-bonding brushes.
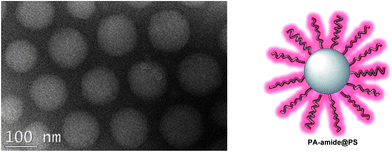 | ||
| Fig. 2 TEM image showing close-packed self-assembly, via hydrogen-bonding, of polystyrene nanoparticles grafted with amide-functionalised polyacrylate brushes (from the article by Chen and Guan, DOI: 10.1039/c3py00078h). | ||
A number of covalent bond-forming processes are also readily reversible and these can, in principle, be exploited in the design and synthesis of thermally-healable materials: such processes are reviewed in this issue by Zhang and Rong (DOI: 10.1039/c3py00005b). Typically they involve Diels–Alder/retro-Diels–Alder reactions, as described here in the work of Sheridan and Bowman (DOI: 10.1039/c2py20960h) and of Pratama, Peterson and Palmese (DOI: 10.1039/c3py00084b), or sulphur-based chemistries such as thiol–disulfide interchange, as reported by Pepels et al. (DOI: 10.1039/c3py00087g).
The strength of a metal–ligand bond lies between that of a full covalent bond and supramolecular interaction, and this type of interaction has been explored in both thermally-induced and photo-stimulated polymer-healing processes. Here, Wang and Urban show that polyethyleneimine cross-linked with Cu(II) provides an elastomeric film that, when damaged, heals rapidly under UV irradiation (DOI: 10.1039/c2py20844j). There is little rise in temperature during the healing process, indicating that the system is genuinely photo-labile (unlike related metal–ligand systems in which UV absorption results in the generation of heat and are thus best described as photo-thermally healable).1
As well as a range of other experimental approaches to self-healing polymers that are reported in the present issue, theoretical issues are addressed by Ciferri (DOI: 10.1039/c3py21156h), and also by Iyer et al., who describe computational studies on self-healing nanoparticle systems related to that shown in Fig. 2 (DOI: 10.1039/c3py00075c).
As guest editors of this themed issue of Polymer Chemistry, we are very grateful to all of those who made it possible by contributing their work to the journal, to the referees involved in assessing the papers, and to the editorial staff of the RSC for their support and assistance.
References
- M. Burnworth, L. Tang, J. R. Kumpfer, A. J. Duncan, F. L. Beyer, G. L. Fiore, S. J. Rowan and C. Weder, Nature, 2011, 472, 334–338 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
