 Open Access Article
Open Access ArticleMesoporous zwitterionic poly(ionic liquid)s: intrinsic complexation and efficient catalytic fixation of CO2†
Sebastian
Soll
a,
Pengfei
Zhang
b,
Qiang
Zhao
a,
Yong
Wang
b and
Jiayin
Yuan
*a
aDepartment of Colloid Chemistry, Max-Planck-Institute of Colloids and Interfaces, D-14476 Potsdam, Germany. E-mail: jiayin.yuan@mpikg.mpg.de; Fax: +49-331-5679502; Tel: +49-331-5679552
bKey Lab of Applied Chemistry of Zhejiang Province, Department of Chemistry, Zhejiang University, Hangzhou, 310028, P.R. China
First published on 1st August 2013
Abstract
Mesoporous poly(ionic liquid)s with zwitterionic structures have been successfully prepared. This synthesis made use of a facile template-free approach via precipitating an organic solution of an imidazolium-based poly(ionic liquid) homopolymer in a straightforward manner into ammonia-containing organic solvents. The complex products have a specific surface area up to 260 m2 g−1, and exist in a robust nanoparticle form. They could serve as active catalysts for the chemical fixation of CO2.
Recently poly(ionic liquid)s or polymerized ionic liquids (PILs) as an innovative type of polyelectrolyte have attracted rapidly increasing attention in the field of polymer and materials science.1–6 They are commonly prepared by conventional or controlled radical polymerization of monomeric ionic liquids (ILs).2,7,8 The presence of an IL moiety in the repeating unit of the polymer chains integrates some IL properties (IL chemistry and physics) into the polymeric architecture. The synergistic effect can positively enable new functions and improve the performance of some polymer materials. This expands the traditional application spectrum of classical polyelectrolytes studied so far. Some application examples of PILs have already been demonstrated very recently, such as polymeric electrolytes, CO2 capture and separation, stationary phases in chromatography, carbon precursors, catalysis, model systems for block copolymer self-assembly, etc.9–19 Among them, the major efforts have been devoted to the chemical design of specific PILs that can deliver superior performance in the bulk and solution.
Porous PILs with enlarged surface area can in principle accelerate the interfacial mass and energy transport, thus representing a promising pathway to amplify material functions even without modifying the chemical structure. Many porous PILs reported to date are macroporous (pore size >50 nm) with specific surface area up to 37 m2 g−1.20–25 Recently via a hard-templating method we synthesized a mesoporous (pore sizes between 2 and 50 nm) PIL with specific surface area up to 220 m2 g−1. This mesoporous PIL demonstrated enhanced CO2 adsorption dynamics and capacity compared to its nonporous bulk counterpart.12 Despite this merit, an obvious drawback associated with the hard-templating method is the inevitable template (usually silica) preparation and removal, which is often time-consuming and hard to scale up. In addition, it involves hazard chemicals, such as fluoride to etch away silica. Therefore, a template-free method to synthesize mesoporous PIL materials was highly desired. This has been recently introduced by us in a simple dissolution–precipitation approach: (1) mixing a PIL with poly(acrylic acid) in DMF to reach a homogeneous blend solution; (2) precipitating the blend solution into NH3-containing organic solvents to trigger the in situ interpolyelectrolyte complexation that subsequently built up porous PIL complexes (denoted as PILCs) with a specific surface area up to 310 m2 g−1.26 It was found that the specific surface area values peaked at a molar ratio of imidazolium cation to carboxylate anion at exactly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The porous structure of the as-synthesized PILC was proven to be highly stable in some organic solvents and was employed as a robust catalyst support. In the follow-up work, we further found that even multi-acid organic compounds could crosslink PILs into porous polyelectrolyte frameworks.27
1. The porous structure of the as-synthesized PILC was proven to be highly stable in some organic solvents and was employed as a robust catalyst support. In the follow-up work, we further found that even multi-acid organic compounds could crosslink PILs into porous polyelectrolyte frameworks.27
Herein we describe a simplified version of this template-free synthetic route towards mesoporous PILCs (mesoPILCs) from a zwitterionic PIL homopolymer as a single structural building component via intrinsic inter-/intrapolyelectrolyte complexation, which features an absolute and favorable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the imidazolium cation to carboxylate anion. In addition, the configuration of the imidazolium cation and the COOH group in the same repeating unit guarantees the molecular homogeneity in the chemical structure all through the porous matrix. One of the as-synthesized mesoPILCs was employed as an efficient and recyclable catalyst in the CO2 cycloaddition reaction.
1 ratio of the imidazolium cation to carboxylate anion. In addition, the configuration of the imidazolium cation and the COOH group in the same repeating unit guarantees the molecular homogeneity in the chemical structure all through the porous matrix. One of the as-synthesized mesoPILCs was employed as an efficient and recyclable catalyst in the CO2 cycloaddition reaction.
The synthetic strategy for the preparation of mesoPILCs is illustrated in Scheme 1. A room temperature IL monomer (compound A in Scheme 1) was first prepared by quaternization of 1-vinyl imidazole with tert-butyl-2-bromoacetate (step i in Scheme 1). The homopolymer PIL (compound B in Scheme 1) was prepared via free radical polymerization of the IL monomer in DMSO at 80 °C using 2,2′-azobis(2-methylpropionitrile) (AIBN) as an initiator (step ii in Scheme 1). The tert-butyl protecting group was detached in the sequential step via trifluoroacetic acid (TFA) treatment (step iii in Scheme 1), introducing the carboxylic acid functionalized PIL (compound C in Scheme 1, denoted as PIL–COOH). Precipitation of the DMF or DMSO solution of PIL–COOH into ammonia-containing non-/low polar solvents (here exemplified with diethyl ether) produced insoluble PILCs (step iv in Scheme 1). It should be mentioned that the present PILC formation process differs itself from previously reported systems, in which always two components were required.26,27
 | ||
| Scheme 1 Chemical structure and synthetic route towards a mesoporous zwitterionic PILC from a homopolymer PIL possessing a COOH function. DEE: diethyl ether. | ||
The success in each synthetic step was confirmed by proton nuclear magnetic resonance (1H-NMR) spectroscopy. As shown in Fig. 1B, after quaternization of 1-vinyl imidazole, an intense peak at 1.4 ppm appeared which was assigned to the 9 protons in the tert-butyl group. The full quaternization was verified by the integration ratio (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of the tert-butyl protons (peak h in spectrum B) to the well-resolved single vinyl proton (peak c in spectrum B) at 5.8 ppm. Following the polymerization, all vinyl protons (peaks a, b and c in spectrum B) eventually vanished and two new broad peaks at 2.6 and 4.1 ppm (spectrum C in Fig. 1) were observed, which could be assigned to the two types of backbone protons (–CH2–C(H)<). The cleavage of the tert-butyl group proceeded quantitatively, as the strong peak at 1.4 ppm disappeared, leaving the remaining peaks actually untouched. All products until this step have good solubility in organic solvents, like DMF or DMSO. The intrinsic inter-/intra-polyelectrolyte complexation occurred when the PIL–COOH solution was dropped into ammonia-containing organic solvents. In this process, the COOH function was immediately deprotonated, which triggered the in situ complexation between the carboxylate anion and the imidazolium cation and built up an ionically crosslinked polymer network. What is very unique in this system is, PIL–COOH acts as the only building component, therefore the complexation takes place only among different PIL–COOH chains (interpolyelectrolyte complexation) or within the same PIL–COOH chain (intrapolyelectrolyte complexation). After the charge neutralization, the polymer became insoluble in many organic solvents and was only partially swollen in DMSO under heating.
1) of the tert-butyl protons (peak h in spectrum B) to the well-resolved single vinyl proton (peak c in spectrum B) at 5.8 ppm. Following the polymerization, all vinyl protons (peaks a, b and c in spectrum B) eventually vanished and two new broad peaks at 2.6 and 4.1 ppm (spectrum C in Fig. 1) were observed, which could be assigned to the two types of backbone protons (–CH2–C(H)<). The cleavage of the tert-butyl group proceeded quantitatively, as the strong peak at 1.4 ppm disappeared, leaving the remaining peaks actually untouched. All products until this step have good solubility in organic solvents, like DMF or DMSO. The intrinsic inter-/intra-polyelectrolyte complexation occurred when the PIL–COOH solution was dropped into ammonia-containing organic solvents. In this process, the COOH function was immediately deprotonated, which triggered the in situ complexation between the carboxylate anion and the imidazolium cation and built up an ionically crosslinked polymer network. What is very unique in this system is, PIL–COOH acts as the only building component, therefore the complexation takes place only among different PIL–COOH chains (interpolyelectrolyte complexation) or within the same PIL–COOH chain (intrapolyelectrolyte complexation). After the charge neutralization, the polymer became insoluble in many organic solvents and was only partially swollen in DMSO under heating.
 | ||
| Fig. 1 1H-NMR spectra (in D2O) of 1-vinyl imidazole (A), monomeric IL (B), its corresponding PIL (C) and the deprotected form PIL–COOH (D). | ||
Fig. 2A shows a typical nitrogen sorption isotherm of the PILC sample prepared by precipitation of a PIL–COOH solution (7 wt%) in DMSO into NH3-containing (0.5 wt%) diethyl ether. It exhibits unambiguously a type-IV isotherm with a hysteresis loop in the p/p0 range from 0.7 to 0.9. The pore size distribution curve (Fig. S1†) indicates that the material is essentially made up of mesopores (6–20 nm in size). The pore structure is robust in organic solvents, such as acetone, THF or acetonitrile, even after several cycles of wet–drying or refluxing, as its characteristics in gas sorption measurements changed little. This is actually expected for such a densely and ionically crosslinked system, in good accordance with other PILC systems prepared previously.26,27 The specific surface area calculated from Brunauer–Emmett–Teller equation (SBET) is 260 m2 g−1.
 | ||
| Fig. 2 (A) Nitrogen sorption isotherms of the mesoPILC product prepared via precipitation of 7 wt% of the PIL–COOH solution in DMSO into diethyl ether with (solid dots) and without ammonia activation (open circles). (B) Plot of specific surface area of the formed mesoPILC products vs. the PIL–COOH concentration in DMSO. | ||
It should be noted that ammonia played a central role in controlling the inter-/intra-polyelectrolyte complexation and pore generation. Without adding ammonia, no porous product formed (Fig. 2A) and the precipitating solid in diethyl ether could be re-dissolved in DMF or DMSO because of no network formation in PIL–COOH. Furthermore, in the case of NH3-containing complexation solvents (in which the complexation takes place), such as diethyl ether as in this case, the SBET values were able to be modulated between 160 and 260 m2 g−1via the PIL–COOH concentration in DMSO. As shown in Fig. 2B, the highest SBET value was obtained at a PIL–COOH concentration of 7 wt%. Below or above this concentration, the SBET value drops. Besides, the complexation solvent quality also plays an important role. Porous materials come up only when the PIL–COOH solution was precipitated into organic solvents of low polarity, such as diethyl ether, hexane (150 m2 g−1) and cyclohexane (174 m2 g−1). Compact and nonporous products were received when complexation was conducted in water, methanol or DMSO in the presence of NH3. Therefore, a poor or non-solvent of PIL–COOH seems to be the prerequisite for preparing mesoPILCs in the current system.
Scanning electron microscopy (SEM) characterization (Fig. 3) provides additional information on the morphology of the mesoPILC product. As can be clearly observed, it exhibits distinctive round-shaped particles stacking onto each other. The particles are 30 to 50 nm in size and exist as a secondary structure motif of the primary aggregates of the ionically locked PIL backbones. Thermogravimetric analysis (TGA) indicated that the first thermal decomposition step took place at 250 °C (Fig. S2†). Because of its nanoparticle shape and good thermal stability, we investigated whether the as-synthesized mesoPILC could work directly as a catalyst. The cycloaddition reaction of CO2 to epoxides, a reaction related to the chemical storage of CO2 by capturing it as a building block in C1 chemistry, was selected as a model reaction. It is worth noting that several halide-containing crosslinked PIL nanoparticles have been recently tested by Xiong et al. and showed active catalytic activity for this reaction.28–31
 | ||
| Fig. 3 SEM images of the mesoPILC product prepared from precipitation of PIL–COOH solution (7 wt% in DMSO) into ammonia-containing diethyl ether. (B) Enlarged view of (A). | ||
The catalytic tests were conducted in an autoclave at 160 °C under 1 MPa CO2. Propylene oxide, styrene oxide and epichlorohydrin were chosen as exemplary substrates. The results are summarized in Table 1. As can be seen, all three substrates can be smoothly transformed into cyclic carbonates with satisfactory conversion (≥93%) when using the mesoPILC nanoparticles as a catalyst. A high selectivity (≥97%) was obtained in the cycloaddition of CO2 with propylene oxide and epichlorohydrin, while a relatively lower one of 87% was found for styrene oxide, which is possibly caused by the enhanced steric hindrance due to the large phenyl ring.
| Entry | Epoxide | Product | T/h | Conv. | Sel. |
|---|---|---|---|---|---|
| 1 |
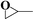
|
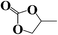
|
6 | 93% | 98% |
| 2 |

|

|
24 | 94% | 87% |
| 3 |

|

|
8 | 99% | 97% |
Further experiments were conducted to examine the recyclability of the porous PILC nanoparticles in the CO2 cycloaddition by using propylene oxide as a model substrate. As shown in Fig. 4, the PILC catalyst could be reused for at least four runs without significant loss of the catalytic activity, which is a prerequisite for practical applications. The average conversion of these four runs is 94%, and a slight deviation (4% in 2nd and 4th runs) is within the experimental error. Meanwhile the catalytic selectivity remains constantly high (≥97%). The overall superior catalytic activity, high selectivity and excellent recyclability of mesoPILCs as a heterogeneous catalyst could be attributed to their excellent structural stability and unique nanoscale morphology. The reaction temperature (160 °C) is far below the thermal decomposition threshold temperature (Fig. S2†). The nanoparticle form and the porous state allow for a rapid diffusion of the reactants easily into the reactive center. At the same time the ionic crosslinking firmly holds the particle structure together in the organic liquid product, facilitating the easy separation by simple filtration from the liquid products.
 | ||
| Fig. 4 The reusability of a mesoPILC catalyst. Reaction conditions: propylene epoxide (3 mL), catalyst: 50 mg, 1 MPa CO2, 150 °C, t = 6 h. | ||
In summary, we have developed a facile synthetic route to convert a zwitterionic PIL homopolymer into a mesoporous PIL complex via precipitating the PIL solution into organic solvents of low polarity. The obtained porous polyelectrolyte networks have nanoparticle morphology and are structurally stable in some organic solvents even under reflux treatment. In addition, they were successfully used as catalysts for the cycloaddition of CO2 to epoxides. From a viewpoint of the chemical structure and application potential, this single-component zwitterionic mesoporous PILC might broaden the current research scope of poly(ionic liquid)s, which is mostly polycation-based.
Acknowledgements
The authors thank the Max Planck Society for the financial support. Y. Wang would like to acknowledge the Partner Group Program of Zhejiang University in China and the Max Planck Society in Germany.Notes and references
- O. Green, S. Grubjesic, S. Lee and M. A. Firestone, Polym. Rev., 2009, 49(4), 339–360 CrossRef CAS.
- J. Yuan and M. Antonietti, Polymer, 2011, 52(7), 1469–1482 CrossRef CAS.
- J. Lu, F. Yan and J. Texter, Prog. Polym. Sci., 2009, 34(5), 431–448 CrossRef CAS.
- D. Mecerreyes, Prog. Polym. Sci., 2011, 36(12), 1629–1648 CrossRef CAS.
- J. Yuan, D. Mecerreyes and M. Antonietti, Prog. Polym. Sci., 2013, 38, 1009–1036 CrossRef CAS.
- M. D. Green and T. E. Long, Polym. Rev., 2009, 49(4), 291–314 CrossRef CAS.
- C. Detrembleur, A. Debuigne, M. Hurtgen, C. Jérôme, J. Pinaud, M. v. Fèvre, P. Coupillaud, J. Vignolle and D. Taton, Macromolecules, 2011, 44(16), 6397–6404 CrossRef CAS.
- M. D. Green, D. Wang, S. T. Hemp, J.-H. Choi, K. I. Winey, J. R. Heflin and T. E. Long, Polymer, 2012, 53(17), 3677–3686 CrossRef CAS.
- Q. Zhao, T.-P. Fellinger, M. Antonietti and J. Yuan, Macromol. Rapid Commun., 2012, 33(13), 1149–1153 CrossRef CAS.
- J. Yuan, A. G. Marquez, J. Reinacher, C. Giordano, J. Janek and M. Antonietti, Polym. Chem., 2011, 2(8), 1654–1657 RSC.
- H. Ohno, Macromol. Symp., 2007, 249–250(1), 551–556 CrossRef.
- A. Wilke, J. Yuan, M. Antonietti and J. Weber, ACS Macro Lett., 2012, 1028–1031 CrossRef CAS.
- J. E. Bara, D. L. Gin and R. D. Noble, Ind. Eng. Chem. Res., 2008, 47(24), 9919–9924 CrossRef CAS.
- J. E. Bara, S. Lessmann, C. J. Gabriel, E. S. Hatakeyama, R. D. Noble and D. L. Gin, Ind. Eng. Chem. Res., 2007, 46(16), 5397–5404 CrossRef CAS.
- F. Zhao, Y. Meng and J. L. Anderson, J. Chromatogr., A, 2008, 1208(1–2), 1–9 CrossRef CAS.
- G. R. Lambertus, J. A. Crank, M. E. McGuigan, S. Kendler, D. W. Armstrong and R. D. Sacks, J. Chromatogr., A, 2006, 1135(2), 230–240 CrossRef CAS.
- J. Yuan, S. Wunder, F. Warmuth and Y. Lu, Polymer, 2012, 53(1), 43–49 CrossRef CAS.
- J. Pinaud, J. Vignolle, Y. Gnanou and D. Taton, Macromolecules, 2011, 44(7), 1900–1908 CrossRef CAS.
- Y. Ye, S. Sharick, E. M. Davis, K. I. Winey and Y. A. Elabd, ACS Macro Lett., 2013, 575–580 CrossRef CAS.
- C. Boyere, A. Favrelle, A. F. Leonard, F. Boury, C. Jerôme and A. Debuigne, J. Mater. Chem. A, 2013, 1, 8479–8487 CAS.
- F. Yan and J. Texter, Angew. Chem., Inter. Ed., 2007, 119(14), 2492–2495 CrossRef.
- J. Huang, C.-a. Tao, Q. An, W. Zhang, Y. Wu, X. Li, D. Shen and G. Li, Chem. Commun., 2010, 46(6), 967–969 RSC.
- X. Hu, J. Huang, W. Zhang, M. Li, C. Tao and G. Li, Adv. Mater., 2008, 20(21), 4074–4078 CrossRef CAS.
- D. England, N. Tambe and J. Texter, ACS Macro Lett., 2012, 1(2), 310–314 CrossRef CAS.
- F. Yan, D. England, H. Gu and J. Texter, Reversibly Porating Coatings? Pinned Spinodal Decompositions, in Smart Coatings III, American Chemical Society, 2010, vol. 1050, pp. 191–207 Search PubMed.
- Q. Zhao, P. Zhang, M. Antonietti and J. Yuan, J. Am. Chem. Soc., 2012, 134(29), 11852–11855 CrossRef CAS.
- Q. Zhao, S. Soll, M. Antonietti and J. Yuan, Polym. Chem., 2013, 4(8), 2432–2435 RSC.
- Y.-B. Xiong, H. Wang, Y.-J. Wang and R.-M. Wang, Polym. Adv. Technol., 2012, 23(5), 835–840 CrossRef CAS.
- Y. Xiong, J. Liu, Y. Wang, H. Wang and R. Wang, Angew. Chem., Int. Ed., 2012, 51, 9114–9118 CrossRef CAS.
- Y. Xiong, Y. Wang, H. Wang and R. Wang, Polym. Chem., 2011, 2(10), 2306–2315 RSC.
- Y. Xiong, H. Wang, R. Wang, Y. Yan, B. Zheng and Y. Wang, Chem. Commun., 2010, 46(19), 3399–3401 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and some characterization of poly(ionic liquid)s are presented. See DOI: 10.1039/c3py00823a |
| This journal is © The Royal Society of Chemistry 2013 |
