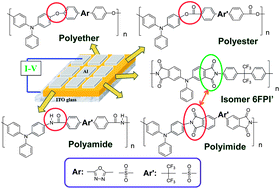
A new functional triphenylamine-based (TPA-based) aromatic polyether (OXPE) derived from 2,5-bis(4-fluorophenyl)-1,3,4-oxadiazole and 4,4′-dihydroxytriphenylamine and polyester (6FPET) derived from 4,4′-(hexafluoroisopropylidene)bis(benzoyl chloride) and 4,4′-dihydroxytriphenylamine were synthesized and used for memory device applications. To get more insight into the relationship between the linkage group and memory behavior, polyamide 6FPA, polyimide 6FPI, and the corresponding isomer 6FPI′ were also synthesized and their memory properties were investigated. The linkage effects of polyether, polyester, polyamide, and polyimide are expected to reveal different retention times of the synthesized polymer memory devices due to their different structural conformations, dipole moments, HOMO, and LUMO energy levels. OXPE, 6FPA, and 6FPET with a non-planar linkage structure but different LUMO energy levels possess SRAM behavior with different retention times, 2 min, 5 min, and 6 min, respectively. 6FPI with a planar linkage group exhibits DRAM property while the corresponding isomer 6FPI′ reveals insulator behavior due to the difficulty in sustaining the charge transfer complex. Furthermore, to illustrate the linkage and acceptor effect systematically, TPA-based sulfonyl-containing polymers with the same linkages were also added into the discussion.