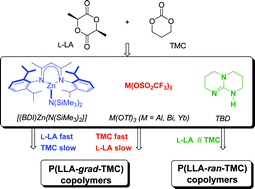
Copolymerization of L-LA and TMC has been achieved with binary systems, associating either a metal-based (pre)catalyst or an organocatalyst with benzyl alcohol (BnOH) acting as a co-initiator and a chain transfer agent. Kinetic monitoring indicated that copolymerizations mediated by the zinc-β-diketiminate system [(BDI)Zn{N(SiMe3)2}]/BnOH proceed by preferential consumption of L-LA first and then of TMC, in contrast to homopolymerizations of the individual monomers that progress roughly at the same rate. In striking contrast, systems based on metal triflates, M(OTf)3/BnOH (M = Al, Bi, Yb), copolymerize TMC faster than L-LA, as established for the first time, while these systems also homopolymerize the individual monomers at about the same rate. Eventually, the [(BDI)Zn{N(SiMe3)2}]/BnOH and M(OTf)3/BnOH systems produce P(LLA-grad-TMC) gradient copolymers featuring PLLA and PTMC blocks of significant length (ca. 10 units with Al-, Yb-; ca. 6 units with Zn-, Bi-based systems), as confirmed by 13C NMR analyses. On the other hand, copolymerizations mediated by the guanidine-based organocatalyst system TBD/BnOH (TBD = 1,5,7-triazabicyclo[4.4.0]dec-5-ene) gave P(LLA-ran-TMC) random copolymers. Optimal control of molar masses and dispersities, along with minimized transesterifications, is achieved with the zinc and ytterbium systems. While metal triflates induce partial decarboxylation of TMC units, the zinc and TBD systems are perfectly chemoselective. Kinetic and microstructural control in random copolymerization of L-LA and TMC can thus be achieved via catalytic tuning.