 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polypyrrole–silver composites prepared by the reduction of silver ions with polypyrrole nanotubes
Jitka Škodováa, Dušan Kopeckýa, Martin Vrňata*a, Martin Vargab, Jan Prokešb, Miroslav Cieslarb, Patrycja Boberc and Jaroslav Stejskalc
aFaculty of Chemical Engineering, Institute of Chemical Technology Prague, 166 28 Prague 6, Czech Republic. E-mail: vrnatam@vscht.cz
bCharles University in Prague, Faculty of Mathematics and Physics, 180 00 Prague 8, Czech Republic
cInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, 162 06 Prague 6, Czech Republic
First published on 5th April 2013
Abstract
Polypyrrole nanotubes were prepared by the oxidation of pyrrole with iron(III) chloride in the presence of methyl orange. They were subsequently used for the reduction of silver ions to silver nanoparticles. The nanotubular form of polypyrrole is compared with the classical globular morphology in its ability to reduce silver ions. Both polypyrrole salts and bases were used in the experiments. The content of metallic silver in the resulting composite, determined by thermogravimetric analysis, was 21–31 wt%. Elemental composition is also discussed on the basis of energy-dispersive X-ray spectroscopy. Contrary to the expectation, the conductivity of polypyrrole nanotubes in salt form, 35.7 S cm−1, was reduced to 20.9 S cm−1 after the incorporation of silver. The presence of silver had generally little effect on the conductivity. The temperature dependence of conductivity reveals that the composites maintain the semiconducting character of polypyrrole and their conductivity increased with increasing temperature. The conductivity of the composites surprisingly increased when the samples were placed in vacuo.
Introduction
The combination of conducting polymers and noble metal nanoparticles has recently become a tool in the preparation of new materials. The composites combining two conducting components, a metal and an organic semiconductor, are expected to exhibit a good level of electrical conductivity, as well as tunable physical, chemical, and responsive properties. Among them, polypyrrole (PPy) and polyaniline (PANI) composites with silver have become frequently studied.1The ability of PPy to reduce silver ions to metallic silver has been reported many times. The paper by Pickup et al.2 is important from a historical point of view. In this early study, the authors illustrated the ability of electrochemically prepared PPy film to reduce silver ions to silver, and this was confirmed in follow-up experiments.3,4 The progress of reaction, the reduction of silver cations on PPy film, was followed by a quartz microbalance.5 This reaction was used especially for silver recovery from solutions of its salt.2 Counter-ions in PPy were demonstrated to have a significant effect on the silver yield,6 because some of them, such as chlorides or, to a certain extent, sulfates, may form insoluble salts with silver cations. Polypyrrole was also deposited on supports, such as sawdust7 or porous carbon8 for recovery purposes. In addition, some studies assumed the complexation of silver ions with nitrogen atoms in PPy,8 rather than the chemical reaction between both species. Polypyrrole was used as-prepared, i.e. in the form of salt. Only exceptionally it was reduced with sodium borohydride at first,9 or converted to PPy base in alkalis. Recent applications concern, e.g., supports for surface-enhanced Raman scattering,10 catalytic reduction of hydrogen peroxide10 or efficient antimicrobial agents.11
Depending on the conditions, the reduction of silver ions with PPy yielded silver particles with sizes below 10 nm,12,13 tens of nanometres or micrometres.10,11,14 Larger objects, such as silver triangles,5 nanosheets15,16 and nanocables,17,18 have also been observed.
The morphology of PPy entering the reaction was also considered, and attention has been paid especially to nanotubes. PPy nanotubes were prepared by the coating of vanadium(V) oxide nanowires with PPy and subsequent dissolution of the template. When exposed to silver nitrate solutions, 4–8 nm silver nanoparticles were produced on their surface and also agglomerated inside the nanotube cavity.19 Aluminium oxide membranes have been used as a hard template for the reduction of silver ions.11 Similar experiments have also been made with PANI nanotubes and silver was occasionally located inside the nanotubes.20,21 In another approach, PPy nanotubes, prepared in the presence of methyl orange as a structure-guiding agent, were immersed in silver nitrate solution and thus decorated on the surface with silver nanoparticles.22–24 In these experiments, however, poly(N-vinylpyrrolidone) has been used as an additive to prevent the aggregation of silver nanoparticles. Another report used similar PPy nanotubes functionalized with carboxyl groups.10
In the present study, PPy nanotubes were prepared also in the presence of methyl orange, but in the absence of any additives and without any modification. Their ability to reduce silver ions is compared with that of the classical globular form of PPy. In addition, both PPy salts and PPy bases have been used in the experiments. Attention has been paid especially to the electrical conductivity of the composites.
Experimental
Preparation of granular PPy and PPy nanotubes
Granular PPy was prepared by the chemical polymerization of pyrrole monomer with iron(III) chloride hexahydrate at an equimolar ratio in water. Iron(III) chloride hexahydrate (3.9 g; 14 mmol) was dissolved in 287 mL of distilled water, then pyrrole (1 mL; 14 mmol) was added. Molar concentrations of both reactants were 50 mM, the total volume of reaction mixture was 288 mL. The stirred reaction mixture was kept at 5 °C for 24 h. The precipitated PPy was separated by filtration, rinsed with acetone and ethanol, and dried at 40 °C in vacuo.Polypyrrole nanotubes were synthesized in a similar manner in the presence of structure-guiding agent, methyl orange, (4-[4-(dimethylamino)phenylazo]benzenesulfonic acid sodium salt).10,22–24 Methyl orange solution in distilled water (287 mL; 2.5 mM) and pyrrole (1 mL) were mixed. Then iron(III) chloride hexahydrate (3.9 g) was dissolved in distilled water (33 mL) and added dropwise over two hours into the reaction solution. Both solutions were cooled to 5 °C before mixing. Thus the molar concentrations of reactants were 45 mM pyrrole, 45 mM iron(III) chloride hexahydrate, and 2.2 mM methyl orange. After 24 h, precipitated PPy nanotubes were separated by filtration, rinsed with water and subsequently purified by Soxhlet extraction to remove residual methyl orange using acetone until the extract was colourless. Finally, extracted PPy was rinsed with ethanol. Polypyrrole nanotubes were dried as above. Parts of PPy salts were converted to the corresponding bases25 by immersion in an excess of 1 M ammonium hydroxide, rinsed with acetone, and dried. Thus obtained PPy powders of salts or bases were immersed in an excess of 0.1 M aqueous solution of silver nitrate and left for 24 h to interact. The solids were collected on a filter, rinsed with water, and dried. Nitric acid is the by-product of these reactions1 and its presence could cause a partial reprotonation of the original PPy bases.
Characterization
A transmission electron microscope (TEM) JEOL JEM 2000 FX was used to assess the morphology. Thermogravimetric analysis (TGA) was performed in a 50 cm3 min−1 air flow at a heating rate of 10 °C min−1 with a Perkin Elmer TGA 7 Thermogravimetric Analyzer. Elemental analysis was done with an elemental analyzer Elementar Vario EL III (Elementar Analysensysteme GmbH) with accuracy <0.1 wt%. Surface composition was characterized by energy-dispersive X-ray spectroscopy (EDS) using a JEOL JXA 50A electron microprobe equipped with a Bruker AXS 4010 XFlash SD detector. The conductivity of the granular PPy was estimated from powder placed between two conducting pistons by a two-probe method with an applied pressure of ca 23 kPa and using a Keithley 6517 electrometer. In contrast to the nanotubes, granular PPy could not be compressed to pellets. The room temperature conductivity of PPy nanotubes was determined on pellets compressed at 700 MPa by a four-point method in the van der Pauw arrangement using a Keithley 220 Programmable Current Source, a Keithley 2010 Multimeter as a voltmeter and a Keithley 705 Scanner equipped with a Keithley 7052 Matrix Card.The temperature dependence of conductivity in the range of 2–318 K, and conductivity under dynamic vacuum (∼units of Pa), were measured on a commercial device Quantum Design PPMS by the conventional four-point method on bar-shaped samples where current probes were placed on the edges of the bar and voltage probes were situated in the middle of the bar.
Results and discussion
Polypyrrole prepared by the oxidation of pyrrole in acidic aqueous media is obtained as a salt. In an idealized case, proposed in analogy with PANI,25 the PPy salts convert to PPy bases when exposed to alkalis, such as ammonium hydroxide (Fig. 1). While in the case of PANI the salt–base transition has been well established, in the case of PPy it is mentioned only rarely and in the course of other studies.26–28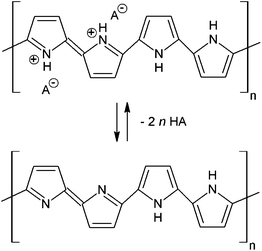 | ||
| Fig. 1 PPy salts convert in alkaline media to the corresponding base. HA is an arbitrary acid, here hydrochloric acid. | ||
Both the PPy salt and PPy base are able to reduce silver cations to silver. A formal reaction scheme of the reduction of silver ions with PPy can be proposed (Fig. 2), again in analogy with a similar reaction involving a closely related polymer, PANI. In the latter case, the emeraldine form of PANI converts to pernigraniline29,30 and silver ions are reduced to silver metal. As the reaction between silver ions and PPy has been proven to take place, an oxidized form of PPy, and an analogous form of pernigraniline in PANI, is likely to exist (Fig. 2).
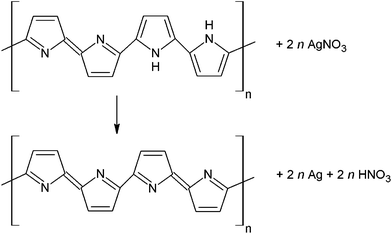 | ||
| Fig. 2 PPy base reduces silver salts, such as silver nitrate, to metallic silver. Nitric acid is a by-product. | ||
Polypyrrole morphology
The classical oxidation of pyrrole with iron(III) chloride in water yields PPy with a globular morphology (Fig. 3a). The introduction of a small amount of some additives, such as p-phenylenediamine, was demonstrated to considerably affect the morphology of PANI.31 The presence of methyl orange in the oxidation of pyrrole results in the formation of nanotubes (Fig. 3b). The nanotubular morphology, i.e. the existence of the cavity, is demonstrated by transmission electron microscopy (Fig. 4). Both PPy morphologies, globules and nanotubes, in their salt and base forms, have been tested for their ability to reduce silver ions.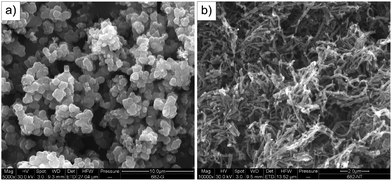 | ||
| Fig. 3 Scanning electron microscopy of (a) granular and (b) nanotubular PPy. | ||
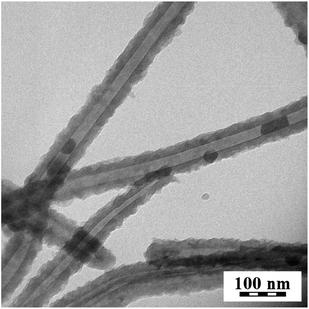 | ||
| Fig. 4 Transmission electron microscopy illustration of nanotubular structure. | ||
Reduction of silver ions with PPy
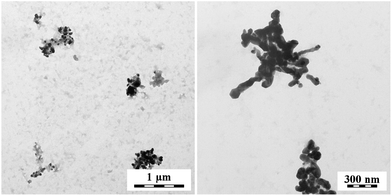
Transmission electron microscopy is a convenient tool in the assessment of PPy–silver composites as it provides a good contrast between both phases (Fig. 5–8). The reaction of globular PPy salt with silver ions generates aggregates of silver nanofibers (Fig. 5). On the contrary, the nanotubular PPy salt produces aggregates of globular nanoparticles (Fig. 6).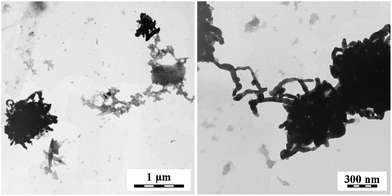 | ||
| Fig. 5 PPy composites obtained by the reduction of silver nitrate with globular PPy salt. Two magnifications. | ||
 | ||
| Fig. 6 PPy composites obtained by the reduction of silver nitrate with nanotubular PPy salt. Two magnifications. | ||
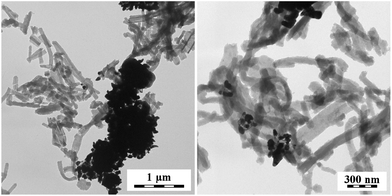
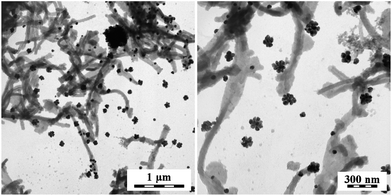
The PPy bases act in a different manner. The globular PPy base enforced the globular morphology to silver (Fig. 7), while PPy nanotubes provide flower-like silver aggregates (Fig. 8). It has to be stressed, that PPy and silver are separated in all cases. The micrographs, however, show just a small part of the sample and need not represent the sample as a whole.
 | ||
| Fig. 7 PPy composites obtained by the reduction of silver nitrate with globular PPy base. Two magnifications. | ||
 | ||
| Fig. 8 PPy composites obtained by the reduction of silver nitrate with nanotubular PPy base. Two magnifications. | ||
Silver content and elemental analysis
The silver content in the composites is an important factor. Stoichiometry according to Fig. 2 expects 45.7 wt% Ag. The silver content (Table 1) can be determined by thermogravimetric analysis of a residue (Fig. 9). The analysis has to be made in air; in nitrogen PPy is carbonized,32,33 but not decomposed, similarly to PANI34,35 and the residue after analysis would be much higher. Even in air, PPy alone leaves a residue of 3.1 or 3.8 wt% for globular and nanotubular forms, respectively, which was subtracted when calculating the silver content, and is associated most likely with iron oxides.| Polypyrrole | Composition [wt%] | Conductivity [S cm−1] | ||||
|---|---|---|---|---|---|---|
| Ag | C | H | N | As prepared | With silver | |
| Nanotubes, salt | 21.1 | 57.7 | 4.6 | 16.2 | 35.7 | 20.9 |
| Nanotubes, base | 15.3 | 61.3 | 4.6 | 17.2 | 0.056 | 1.38 |
| Globular, salt | 21.0 | 56.0 | 4.4 | 16.5 | 0.012 | 0.028 |
| Globular, base | 30.6 | 61.5 | 4.3 | 17.4 | 3.7 × 10−5 | 1.1 × 10−4 |
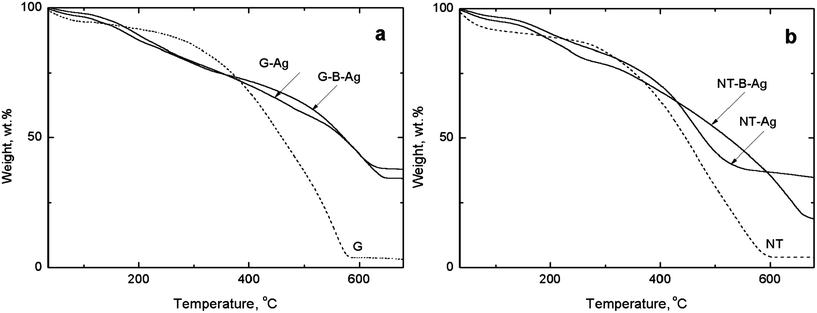 | ||
| Fig. 9 Thermogravimetric analysis of (a) globular and (b) nanotubular PPy and the composites of its salt and base forms with silver. | ||
The results of the elemental analysis of C, H, and N (Table 1) refer only to the PPy part of the composite, but not to the composite as a whole. After the conversion of the PPy salts to bases, the relative contents of carbon and nitrogen increase, due to the removal of inorganic acid (Fig. 1). The content of sulfur (associated with the potential presence of methyl orange) was below the detection limit.
Elemental composition by EDS
The subsequent analysis has been limited to PPy nanotubes only, as they are a more interesting object from the morphological point of view. EDS is a straightforward way to prove the presence of silver in the composites and to determine its content (Table 2). From this point of view, nanotubular PPy salt seems to be a more efficient reducing agent (compared with PPy base) of silver ions. A more critical observer, however, may object that a part of silver in the composite with PPy salt may be present as silver chloride, due to chloride ions originating from iron(III) chloride oxidant. Indeed, after the deprotonation of the composite with ammonium hydroxide, which would dissolve silver chloride, the content of silver would be reduced, as observed. Furthermore, the previous study36 devoted to a similar study on PANI protonated with various acids (H+A−) revealed that the decreasing solubility product of the corresponding silver salt (Ag+A−) correlates with the increasing content of silver in the resulting composite, either in the form of insoluble salt or in metallic form. On the other hand, there was no connection between the presence of silver salt and conductivity of the composite.| Sample | Ag | C | N | O | S | Cl | Fe | C/N | O/N | Cl/N |
|---|---|---|---|---|---|---|---|---|---|---|
| PPy | — | 58.1 | 19.3 | 11.3 | 2.5 | 8.0 | 0.8 | 3.54 | 0.51 | 0.16 |
| PPy/Ag | 13.7 | 45.6 | 14.9 | 17.9 | 2.5 | 4.8 | 0.6 | 3.57 | 1.05 | 0.13 |
| PPy base | — | 60.5 | 20.4 | 16.3 | 1.5 | 0.9 | 0.3 | 3.45 | 0.70 | 0.02 |
| PPy base/Ag | 8.5 | 48.5 | 18.4 | 21.2 | 1.7 | 1.6 | 0.1 | 3.07 | 1.01 | 0.03 |
The content of silver determined by EDS (Table 2) is considerably lower than by TGA (Table 1). This is due to the fact that EDS characterizes especially the composite surface, which may differ in silver content from the bulk materials characterized by TGA. It has often been observed that silver particles are coated with PPy1,37 and this may reduce the silver participation observed by EDS.
Polypyrrole formulae shown in Fig. 1 and 2 are idealized but serve well for the discussion of many PPy properties. A more realistic view has to account for the presence of oxygen atoms in the PPy structure. While the C/N atomic ratio is lower than the theoretical value of 4, as much as one oxygen atom per nitrogen atom seem to be present in reality (Table 2). This is probably a similar situation as with polyaniline, in which the quinonoid and semiquinonoid constitutional units have to be considered38 in addition to the classical concept based entirely on nitrogen-based structures.
The presence of sulfur atoms in the composites (Table 2) suggests the presence of methyl orange, which could not be removed completely, even after exhaustive extraction with acetone. The content of this dye is reduced after deprotonation with ammonium hydroxide but can still be identified in PPy bases. The sulfonate group in methyl orange may also contribute to the oxygen content.
The fact that PPy constitutes a salt with hydrochloric acid (Fig. 1) produced by the hydrolysis of oxidant, iron(III) chloride, is well illustrated by the presence of chlorine atoms (Table 2). The stoichiometry shown in Fig. 1 assumes the Cl/N ratio of 0.5. A considerably lower value is observed in the EDS experiment (Table 2). This can be explained by various means, the washing of samples with water and subsequent partial deprotonation seems to be the most plausible reason.
The deprotonation of PPy salt to PPy base after the treatment with ammonium hydroxide solution is well documented by the reduction of the content of chloride counter-ions (Table 2, Fig. 1). The fact that the oxidized form of PPy in PPy–silver composites has a lower degree of protonation is also supported by EDS (Table 2). The observation of a small amount of iron atoms suggests the presence of complex FeCl4− counter-ions in addition to simple chlorides.
Conductivity
The conductivity of the PPy nanotubes, 35.7 S cm−1, is considerably higher than that of the globular form (Table 1) and also of other conducting polymers, such as polyaniline.30 This value was confirmed in repeated experiments. The deposition of silver reduced the conductivity PPy nanotubes (Table 1) due to the expected oxidation of PPy (Fig. 2).It has been reported that the conductivity of PPy increased when its preparation was carried out in the presence of anionic surfactants bearing a sulfonic group.39,40 Molecules of water-soluble organic dyes are generally composed of a large light-absorbing system of conjugated double bonds and ionic groups, such as a sulfonic group, that ensure the solubility. They therefore resemble surfactants from a colloidal point of view. Methyl orange, a molecule having also a large organic part and a terminal sulfonic group, may thus play a similar role as surfactants and be responsible for good conduction.
In addition to an exceptionally high level of conductivity, PPy nanotubes maintain a good level of conductivity, 0.056 S cm−1 (Table 1), after treatment with ammonia solutions and consequent conversion to PPy base (Fig. 1). This is an even higher value than the conductivity of PPy salt in globular form, as confirmed in repeated experiments. This is an important observation, especially for potential applications in biomedicine, such as hyperthermal cancer therapy,41 where a good level of conductivity has to be maintained as physiological pH ≥ 7. In this case, the incorporation of silver resulted in a moderate increase in the conductivity to 1.38 S cm−1 (Table 1), which would be an additional benefit of PPy–silver composites in these types of applications.
Temperature and time dependence of conductivity
Polypyrrole nanotubes exhibit semiconducting behaviour, as obvious from the temperature dependence of conductivity (Fig. 10) where conductivity increases with increasing temperature, with a large conductivity ratio σ(300 K)/σ(2 K) ≈ 128 for PPy salt nanotubes alone and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 for the composite with silver, respectively, where measurements could be performed down to 2 K. This ratio was σ (300 K)/σ (77 K) ∼ 41
700 for the composite with silver, respectively, where measurements could be performed down to 2 K. This ratio was σ (300 K)/σ (77 K) ∼ 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for PPy base and 54 for the composite with silver, respectively, where resistance was too high to be determined at 2 K. Such a high value of ratio is typical for highly disordered materials at the insulator side far away from the metal–insulator transition.42 The amount of silver in the composites, i.e. 15–30 wt% (Table 1), is then not sufficient for the formation of metallic conducting pathways and, from this point of view, the composites lie in the region below any percolation threshold. Silver nanoparticles thus have probably only a secondary influence on the charge transport properties of composites leaving the key role to the conducting polymer matrix in a similar way as it was observed in PANI–silver composites.43 However, its effect is not easy to understand since, in the case of nanotubular PPy base, silver enhances electrical conductivity and decreases its temperature dependence while for nanotubular PPy salt the effect is exactly opposite, but much smaller. From the TEM study we know that there are differences in morphology that can give at least intuitive insight. Silver nanoparticles form relatively uniformly dispersed small clusters outside the nanotubes (Fig. 8). Then, silver possibly can participate in transport via tunnelling between these metallic islands as it was proposed for granular metals.44 On the other hand, large clusters formed close to nanotubes were found for salts (Fig. 6), but their contribution to transport may be negligible and overall conductivity is probably decreased by the oxidation of PPy salt. Further and deeper analysis and measurements, e.g., magnetoconductance, are essential for the understanding of the charge transport in such a complex system and will be reported in a separate paper.
000 for PPy base and 54 for the composite with silver, respectively, where resistance was too high to be determined at 2 K. Such a high value of ratio is typical for highly disordered materials at the insulator side far away from the metal–insulator transition.42 The amount of silver in the composites, i.e. 15–30 wt% (Table 1), is then not sufficient for the formation of metallic conducting pathways and, from this point of view, the composites lie in the region below any percolation threshold. Silver nanoparticles thus have probably only a secondary influence on the charge transport properties of composites leaving the key role to the conducting polymer matrix in a similar way as it was observed in PANI–silver composites.43 However, its effect is not easy to understand since, in the case of nanotubular PPy base, silver enhances electrical conductivity and decreases its temperature dependence while for nanotubular PPy salt the effect is exactly opposite, but much smaller. From the TEM study we know that there are differences in morphology that can give at least intuitive insight. Silver nanoparticles form relatively uniformly dispersed small clusters outside the nanotubes (Fig. 8). Then, silver possibly can participate in transport via tunnelling between these metallic islands as it was proposed for granular metals.44 On the other hand, large clusters formed close to nanotubes were found for salts (Fig. 6), but their contribution to transport may be negligible and overall conductivity is probably decreased by the oxidation of PPy salt. Further and deeper analysis and measurements, e.g., magnetoconductance, are essential for the understanding of the charge transport in such a complex system and will be reported in a separate paper.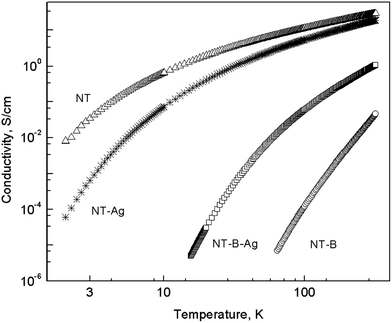 | ||
| Fig. 10 Temperature dependence of the conductivity of the PPy nanotubes in salt and base (B) forms and of their composites with silver. | ||
The effect of dynamic vacuum, in particular moisture removal, on conductivity was also studied (Fig. 11). It turned out to be surprisingly positive, but at the same time it is almost negligible in contrast to other conducting polymer systems, for instance, to recently published results for PANI hydrochloride pellets where moisture is believed to influence conductivity more.45
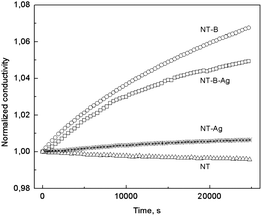 | ||
| Fig. 11 Time evolution of conductivity normalized to the initial value for PPy nanotubes in dynamic vacuum (units of Pa). | ||
Conclusions
(1) Polypyrrole is able to reduce silver ions to metallic silver. This applies both to granular and nanotubular forms of PPy, and both to PPy salts and PPy bases.(2) Polypyrrole salt produces other silver morphologies than the PPy base does. The content of silver is higher in the composites prepared from PPy salts than from PPy bases. Clusters of silver nanoparticles are generated outside PPy. Silver is not produced inside nanotubes.
(3) When PPy salt containing chloride counter-ions reacts with silver nitrate solution, one has to admit that a certain part of silver ions is rather precipitated in the form of water-insoluble silver chloride than reduced into metallic silver.6
(4) The conductivity of PPy nanotubes is high, 35.7 S cm−1, and is moderately reduced by the incorporation of silver. PPy nanotubes maintain a good level of conductivity, 0.056 S cm−1, even after deprotonation with ammonia solution. In the latter case, the presence of silver increased the conductivity to 1.38 S cm−1. This is a high level when considering the potential operation under alkaline conditions.
(5) All PPy samples exhibit semiconducting behaviour and very high temperature resistivity ratio. Silver is believed to have only a secondary role in charge transport in PPy salts. In less conducting PPy bases, it enhances the charge transport. Further, the electrical properties of PPy salts are very stable against applied dynamic vacuum, especially against potential moisture removal.
Acknowledgements
The authors thank the Czech Grant Agency (P108/11/1298, P108/12/P802, and 13-00270S) for financial support. The participation of M. Varga was supported by the grant SVV–2013–267305. The authors would also like to acknowledge the assistance of Mrs E. Šantavá from the Joint Laboratory for Magnetic Studies in Prague with the conductivity measurements at cryogenic temperatures.Notes and references
- J. Stejskal, Chem. Pap., 2013, 67, 814–818 CrossRef CAS.
- N. L. Pickup, J. S. Shapiro and D. K. Y. Wong, Anal. Chim. Acta, 1998, 364, 41–51 CrossRef CAS.
- M. Ocypa, M. Ptasińska, A. Michalska, K. Maksymiuk and E. A. H. Hall, J. Electroanal. Chem., 2006, 596, 157–168 CrossRef CAS.
- Y. Tian, Z. Q. Li, K. Shi and F. L. Yang, Sep. Sci. Technol., 2008, 43, 3891–3901 CrossRef CAS.
- M. M. Ayad and E. Zaki, Appl. Surf. Sci., 2009, 256, 787–791 CrossRef CAS.
- R. Dimeska, P. S. Murray, S. F. Ralph and G. G. Wallace, Polymer, 2006, 47, 4520–4530 CrossRef CAS.
- R. Ansari and A. F. Delavar, J. Iran. Chem. Soc., 2008, 5, 657–668 CrossRef CAS.
- M. Choi and J. Jang, J. Colloid Interface Sci., 2008, 325, 287–289 CrossRef CAS.
- F. M. Kelly, J. H. Johnston, T. Borrmann and M. J. Richardson, Eur. J. Inorg. Chem., 2007, 2007, 5571–5577 CrossRef.
- Y. J. Peng, L. H. Qiu, C. T. Pan, C. C. Wang, S. M. Shang and F. Yan, Electrochim. Acta, 2012, 75, 399–405 CrossRef CAS.
- E. Park, H. Kim, J. Song, H. Oh, H. Song and J. Jang, Macromol. Res., 2012, 20, 1096–1101 CrossRef CAS.
- E. Pinter, R. Patakfalvi, T. Fulei, Z. Gingl, I. Dekany and C. Visy, J. Phys. Chem. B, 2005, 109, 17474–17478 CrossRef CAS.
- C. Visy, E. Pinter, T. Fulei and R. Patakfalvi, Synth. Met., 2005, 152, 13–16 CrossRef CAS.
- L. Kabir, A. R. Mandal and S. K. Mandal, J. Exp. Nanosci., 2008, 3, 297–305 CrossRef CAS.
- W. Q. Wang, G. Q. Shi and R. F. Zhang, J. Mater. Sci., 2009, 44, 3002–3005 CrossRef CAS.
- W. Q. Wang and R. F. Zhang, Synth. Met., 2009, 159, 1332–1335 CrossRef CAS.
- A. H. Chen, K. Kamata, M. Nakagawa, T. Iyoda, H. Q. Wang and X. Y. Li, J. Phys. Chem. B, 2005, 109, 18283–18288 CrossRef CAS.
- M. N. Nadagouda and R. S. Varma, Macromol. Rapid Commun., 2007, 28, 2106–2111 CrossRef CAS.
- X. Y. Zhang and S. K. Manohar, J. Am. Chem. Soc., 2005, 127, 14156–14157 CrossRef CAS.
- J. Stejskal, M. Trchová, L. Brožová and J. Prokeš, Chem. Pap., 2009, 63, 77–83 CrossRef CAS.
- M. Trchová and J. Stejskal, Synth. Met., 2010, 160, 1479–1486 CrossRef.
- X. M. Yang, L. Li and F. Yan, Chem. Lett., 2010, 39, 118–119 CrossRef CAS.
- X. M. Yang, L. Li and F. Yan, Sens. Actuators, B, 2010, 145, 495–500 CrossRef CAS.
- X. M. Yang, L. A. Li and Y. Zhao, Synth. Met., 2010, 160, 1822–1825 CrossRef CAS.
- N. V. Blinova, J. Stejskal, M. Trchová, J. Prokeš and M. Omastová, Eur. Polym. J., 2007, 43, 2331–2341 CrossRef CAS.
- O. A. Andreeva and L. A. Burkova, Phys. Solid State, 2011, 53, 1927–1932 CrossRef CAS.
- P. P. Jeeju, S. J. Varma, P. A. F. Xavier, A. M. Sajimol and S. Jayalekshmi, Mater. Chem. Phys., 2012, 134, 803–808 CrossRef CAS.
- V. T. Truong, P. J. McMahon and A. R. Wilson, J. Polym. Sci., Part B: Polym. Phys., 2012, 50, 624–630 CrossRef CAS.
- J. Stejskal, P. Kratochvíl and A. D. Jenkins, Polymer, 1996, 37, 367–369 CrossRef CAS.
- J. Stejskal, I. Sapurina and M. Trchová, Prog. Polym. Sci., 2010, 35, 1420–1481 CrossRef CAS.
- J. Stejskal, I. Sapurina, J. Prokeš and J. Zemek, Synth. Met., 1999, 105, 195–202 CrossRef CAS.
- J. Azadmanjiri, K. Suzuki, C. Selomulya, A. Amiet, J. D. Cashion and G. P. Simon, J. Nanopart. Res., 2012, 14, 1–11 CrossRef.
- K. Y. Park, J. H. Jang, J. E. Hong and Y. U. Kwon, J. Phys. Chem. C, 2012, 116, 16848–16853 CAS.
- M. Trchová, E. N. Konyushenko, J. Stejskal, J. Kovářová and G. Ćirić-Marjanović, Polym. Degrad. Stab., 2009, 94, 929–938 CrossRef.
- M. Trchová, I. Šeděnková and J. Stejskal, Chem. Pap., 2012, 66, 415–445 CrossRef.
- J. Stejskal, M. Trchová, J. Kovářová, L. Brožová and J. Prokeš, React. Funct. Polym., 2009, 69, 86–90 CrossRef CAS.
- M. Chang and E. Reichmanis, Colloid Polym. Sci., 2012, 290, 1913–1926 CAS.
- J. Stejskal and M. Trchová, Polym. Int., 2012, 61, 240–251 CrossRef CAS.
- M. Omastová, M. Trchová, J. Kovářová and J. Stejskal, Synth. Met., 2003, 138, 447–455 CrossRef.
- M. Omastová, M. Trchová, H. Pionteck, J. Prokeš and J. Stejskal, Synth. Met., 2004, 143, 153–161 CrossRef.
- K. Yang, H. Xu, L. Cheng, C. Y. Sun, J. Wang and Z. Liu, Adv. Mater., 2012, 24, 5586–5592 CrossRef CAS.
- C. O. Yoon, M. Reghu, D. Moses and A. J. Heeger, Phys. Rev. B: Condens. Matter, 1994, 49, 10851–10863 CrossRef CAS.
- P. Bober, J. Stejskal, M. Trchová and J. Prokeš, Polymer, 2011, 52, 5947–5952 CrossRef CAS.
- B. Abeles, P. Sheng, M. D. Coutts and Y. Arie, Adv. Phys., 1975, 24, 407–461 CrossRef CAS.
- J. Prokeš, M. Varga, I. Křivka, A. Rudajevova and J. Stejskal, J. Mater. Chem., 2011, 21, 5038–5045 RSC.
| This journal is © The Royal Society of Chemistry 2013 |
