 Open Access Article
Open Access ArticleDynamically functionalized polymersomes via hydrazone exchange†
René P.
Brinkhuis
,
Frank
de Graaf
,
Morten Borre
Hansen
,
Taco R.
Visser
,
Floris P. J. T.
Rutjes
and
Jan C. M.
van Hest
*
Radboud University Nijmegen, Institute for Molecules and Materials, Heyendaalseweg 135, 6525 AJ, Nijmegen, The Netherlands. E-mail: j.vanhest@science.ru.nl; Web: http://www.molchem.science.ru.nl Fax: +31 0243653393
First published on 12th November 2012
Abstract
Polymersomes composed of block copolymers of which the blocks are coupled via a hydrazone moiety are shown to exchange surface PEG chains with the environment via an aniline-catalyzed transimination under equilibrium conditions. This methodology is used to functionalize polymersomes with a different inner and outer moiety in a dynamic covalent way. Secondly, the exchange of surface properties is also demonstrated between differently functionalized polymersomes. These results, therefore, open new routes to the design of complex vesicular surfaces by dynamic exchange.
Polymersomes, or polymeric vesicles, can be regarded as the polymeric analogues of liposomes. Due to the higher molecular weight of the building blocks, polymersomes are considerably tougher and less permeable than their low molecular weight counterparts.1 As a result, polymersomes have an interesting potential for a range of applications such as drug delivery2,3 and in vivo imaging.4,5 For in vivo applications, excellent control over stability and release properties is required, as well as the possibility to functionalize the carrier with specific targeting moieties. An appreciable amount of research has focused on the functionalization of polymersome surfaces6 with enzymes, peptides7 and antibodies.2,8 However, all of these reported functionalization strategies are irreversible or make use of non-covalent interactions.6,9 In contrast, one can also imagine that polymeric vesicles can be functionalized via covalent bonds that are reversible in nature.
Reversible covalent bonds are in (or can be brought to) an equilibrium state, meaning that the bond is reversibly formed and broken as exploited in dynamic combinatorial chemistry.10 In a recent example, Minkenberg et al.11 used this reversible chemistry to spontaneously form amphiphiles that evolved to the thermodynamically most favourable supramolecular composition. If this chemistry is also applicable to preformed polymeric vesicles, it would allow the functionalization of polymersomes in a dynamic and modular way, in which one sample can be (re)-functionalized with a variety of different compounds, while keeping control over vesicle size and stability. Covalent bonds that have the potential to be brought into equilibrium have also been introduced into polymersomes. However, until now, the reversibility has only been used for (triggered) disruption,12–14 unidirectional surface functionalization15 or drug release.16,17
Recently, we showed that the colloidal stability of polymersomes can be controlled by the introduction of a hydrazone bond between the hydrophilic and hydrophobic parts of the constituent block copolymers. The polymeric vesicles were stable at physiological pH, but upon lowering the pH, lost their colloidal stability due to hydrolysis of the hydrazone moiety.18 Simultaneously, a similar system was independently reported by He et al.19 In both studies, lowering the pH was used as a trigger to fully hydrolyze the hydrazone bond in the block copolymer, thus removing the PEG periphery.
At neutral pH, hydrazone bonds are equilibrium structures which favour bond formation as depicted in Fig. 1a. However, bond formation and dissociation are very slow. A method to induce faster dynamics in this equilibrium, without shifting the position, proceeds by the addition of a catalyst, aniline.20 Following this methodology, Dirksen et al.21–23 showed that aniline-catalysed hydrazone exchange between peptidic fragments is 70 fold faster than for the non-catalyzed reaction.
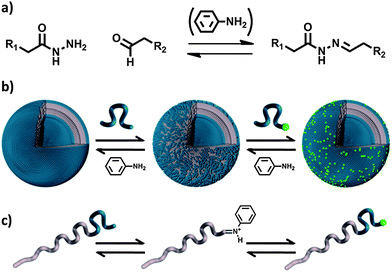 | ||
| Fig. 1 (a) Equilibrium between hydrazide, aldehyde and hydrazone, of which the rate constant can be enhanced by the addition of aniline. (b) After removing a PEG chain from the polymersome surface, either a PEG chain from solution or the original PEG chain can attach. (c) Presentation of the exchange on the block copolymer level, expressing the role of aniline. | ||
Combining our previous work and the herein described aniline catalysed hydrazone exchange on polymersome surfaces allows the design of vesicular architectures with controlled stability, PEGylation and complex membrane functionalities in one single approach.
In this paper, we report the use of an aniline-catalyzed hydrazone equilibrium in the design of polymersomes with dynamic covalent surfaces. We form polymersomes of a hydrazone coupled amphiphilic block copolymer, and make use of the aniline-catalyzed hydrazone equilibrium to exchange surface functionalities with the surrounding solution, as illustrated in Fig. 1b and c. In this way, polymeric vesicles with a different inner and outer functionality can be formed. Finally, we show that the exchange of surface properties between polymersomes is possible, following the same approach. This methodology therefore opens new routes to the design of complex polymeric membranes, starting from simple polymersome scaffolds.
Experimental
Materials and instrumentation
BCN dyes SX-1030 and SX-1031 were purchased from Synaffix. All other reagents were at least 98% pure and used without further purification. Tetrahydrofuran (THF) (ACROS ORGANICS, 99+% extra pure, stabilized with BHT) was distilled under argon from sodium/benzophenone. Polymersome extrusions were performed using 200 nm filters (Acrodisc 13 mm Syringe Filter, 0.2 μm Nylon membrane).MilliQ water was obtained from a Labconco water pro PS system. Particle size distributions were measured on a Malvern instruments Zetasizer Nano-S. Fluorescence intensity measurements were performed on a TECAN Infinite 200 PRO plate reader. Confocal Laser Scanning Microscopy (CLSM) images were recorded on a Leica DM IRE2 TCS SP2 AOBS inverted microscope. An argon laser and a Vioflame diode laser were used to excite the different fluorophores.
Polymer synthesis
Details on the synthesis of the block copolymers as used in this study can be found in the ESI.†Polymersomes
 | ||
| Fig. 2 Dynamic functionalization of polymeric vesicles. (a) Aniline-catalyzed hydrazone surface exchange on polymeric vesicles (black) is faster than non-catalyzed exchange (blue). If no hydrazone is present in the block copolymer, surface exchange is not possible (red). Vesicles were stable and did not change in size during the exchange experiment: 0 hours (b) and 30 hours (c) as determined by DLS. | ||
Dynamic exchange experiments
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 as described above. The experiment was started by mixing 3 mL of polymersomes (0.4 mM), 3 mg of 1 (1.0 mM) and 1.7 mg aniline (6.0 mM).
5 as described above. The experiment was started by mixing 3 mL of polymersomes (0.4 mM), 3 mg of 1 (1.0 mM) and 1.7 mg aniline (6.0 mM).
At different time points, a 600 μL sample was withdrawn and purified over a Sephadex G200 column to remove free PEG and aniline (eluting in MilliQ). The average size at a fixed attenuator and measurement position was determined by DLS and, if necessary, the samples were diluted. No significant changes in size and polydispersity in time were observed. Samples were prepared in a 96 well plate (200 μL) and measured in a plate reader. Both the dansyl and rhodamine probes were selectively visualized subsequently (dansyl excitation 340 nm, emission 540 nm; rhodamine excitation 540 nm, emission 592 nm).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, as described above. The experiment was started by mixing 600 μL of polymersomes (0.4 mM), 0.5 mg 2 (0.56 mM) and 3 mg aniline (50 mM). After 24 hours a sample was purified over a Sephadex G200 column eluting with MilliQ water. The average size at a fixed attenuator and measurement position was determined by DLS, and no significant change in size and polydispersity was observed. The emission spectra of both fluorophores were determined simultaneously in the plate reader (480 nm excitation). For the CLSM experiment both fluorophores were excited with a 476 nm laser. Simultaneously, rhodamine was selectively visualized by recording the emission between 580 and 610 nm, and fluorescein was recorded between 500 and 530 nm (Fig. 3 and S7†).
5, as described above. The experiment was started by mixing 600 μL of polymersomes (0.4 mM), 0.5 mg 2 (0.56 mM) and 3 mg aniline (50 mM). After 24 hours a sample was purified over a Sephadex G200 column eluting with MilliQ water. The average size at a fixed attenuator and measurement position was determined by DLS, and no significant change in size and polydispersity was observed. The emission spectra of both fluorophores were determined simultaneously in the plate reader (480 nm excitation). For the CLSM experiment both fluorophores were excited with a 476 nm laser. Simultaneously, rhodamine was selectively visualized by recording the emission between 580 and 610 nm, and fluorescein was recorded between 500 and 530 nm (Fig. 3 and S7†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (rhodamine-labeled) (10 mL, 0.4 mM); P2 was formed from 6 and 7 in a ratio of 9
1 (rhodamine-labeled) (10 mL, 0.4 mM); P2 was formed from 6 and 7 in a ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (same as P1, but no hydrazone bonds) (10 mL, 0.4 mM); P3 was formed from 3 and 5 in a ratio of 8
1 (same as P1, but no hydrazone bonds) (10 mL, 0.4 mM); P3 was formed from 3 and 5 in a ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (maleimide, but no fluorescent label) (10 mL, 0.4 mM).
2 (maleimide, but no fluorescent label) (10 mL, 0.4 mM).
P3 polymersomes were functionalized with tat-peptide; 1.1 mg tat (∼0.5 equiv towards maleimides) was dissolved in 1 mL MilliQ and 5 mg TCEP gel (Piercenet) was added. The mixture was incubated for 30 minutes, after which the solution was filtered and added to the full 10 mL of maleimide-functional polymersomes P3. Residual tat-peptide was removed by spin column (4000 rpm, 0.1 nm pores) until the eluate showed negative on Kaiser test (ninhydrin for free amines). The total volume was adjusted to 10 mL to have equal concentrations compared to P1 and P2. Next, three samples to test polymersome–polymersome surface exchange were prepared as follows:
(A) 600 μL P1 + 600 μL P3 + 1 mg aniline (1.2 mL sample; 0.4 mM in polymer and 9 mM in aniline).
(B) 600 μL P2 + 600 μL P3 + 1 mg aniline (negative control) (1.2 mL sample; 0.4 mM in polymer and 9 mM in aniline).
(C) 600 μL P1 and 0.5 mg aniline (negative control) (0.6 mL sample; 0.4 mM in polymer and 9 mM in aniline).
After 16 hours, all samples were purified over a Sephadex G200 column to remove any free PEG and aniline (elution with MilliQ). The opaque fractions were combined and used in the HeLa cell studies.
Cell adhesion studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well. At the time of the experiment, cells had grown to approximately 50% confluence. The polymersome samples (20 μL) were diluted with Dulbecco's Modified Eagle Medium DMEM (380 μL) and added to the cells, which were incubated for 1.5 h at 37 °C. The cells were washed with DMEM (3 × 400 μL) and imaged immediately. Confocal laser scanning microscopy was performed using a Leica Microsystems TCS SP2 AOBS system (Mannheim, Germany). Excitation of rhodamine was achieved with an argon laser [488 nm (47%), 514 nm (39%) and 561 nm (36%)], and the resulting emission was acquired between 575 and 725 nm as an average of four scans.
000 cells per well. At the time of the experiment, cells had grown to approximately 50% confluence. The polymersome samples (20 μL) were diluted with Dulbecco's Modified Eagle Medium DMEM (380 μL) and added to the cells, which were incubated for 1.5 h at 37 °C. The cells were washed with DMEM (3 × 400 μL) and imaged immediately. Confocal laser scanning microscopy was performed using a Leica Microsystems TCS SP2 AOBS system (Mannheim, Germany). Excitation of rhodamine was achieved with an argon laser [488 nm (47%), 514 nm (39%) and 561 nm (36%)], and the resulting emission was acquired between 575 and 725 nm as an average of four scans.
Results and discussion
In Scheme 1, the basic building blocks for the self-assembly of polymersomes and the exchange reactions are depicted. Details on the synthetic procedures can be found in the ESI.† We chose to work with the amphiphilic block copolymer polybutadiene-block-poly(ethylene glycol) for two reasons. Firstly, this block copolymer is generally considered to be biocompatible and, secondly, the low glass transition temperature of polybutadiene allows for extrusion and therefore resizing of the polymersomes. This allowed us to prepare well-defined monodisperse samples, thereby making comparison of their properties relatively straightforward. PEG derivatives 1 and 2 contained a hydrazide moiety and were partially labeled with fluorescent dansyl and fluorescein (DY495) markers respectively. Amphiphilic block copolymer 3 contained a hydrazone bond between both blocks and no functional end group. Block copolymer 4 was partially functionalized with rhodamine (DY549) and block copolymer 5 was functionalized with a maleimide end-group. Finally, as a negative control the stable amphiphilic block copolymers 6 and 7 were employed. These polymers are direct analogues of 3 and 4, differing only in the connection between both blocks.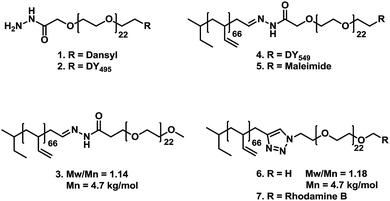 | ||
| Scheme 1 Overview of polymers used in this study. Polymers 1 and 2 are PEG derivatives, both containing a hydrazide moiety and both are labeled with dansyl or fluorescein (DY495) respectively. Polymers 3, 4 and 5 are amphiphilic block copolymers of polybutadiene-b-poly(ethylene glycol), containing a hydrazone linker between both blocks. Polymers 4 and 5 are functionalized with a rhodamine dye (DY549) and a maleimide end group respectively. Finally, block copolymers 6 and 7 do not contain a hydrazone moiety. Polymer 6 contains no functional end group, and 7 is partially labeled with rhodamine B. Both are included as negative controls. | ||
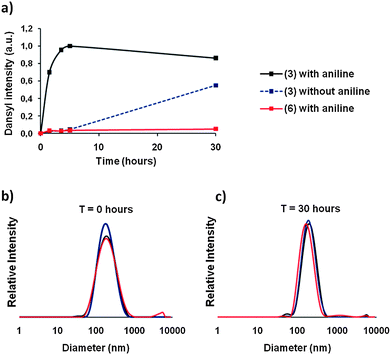
We prepared polymersomes derived from 3 and 6 with a diameter of 220 nm. Thus, polymersomes from 3 contained a hydrazone moiety, while polymersomes from 6 did not, and therefore served as a negative control. After the polymersomes were formed, the fluorescent PEG derivative 1 was added to all samples. To initiate the exchange of surface PEG for the dansyl functionalized PEG (1) in solution, aniline (12 equiv.) was added as a catalyst to the polymersomes formed from 6 and half of the polymersomes formed from 3. To the other half of this sample no catalyst was added, to serve as the second negative control. For each time point a fraction of the samples was withdrawn and purified by size exclusion chromatography (SEC) to separate aniline and unbound PEG from the polymersomes. Next, the fluorescence intensity of the polymersome fraction was measured in a plate reader as plotted in Fig. 2a (340 nm (excitation), 560 nm (emission)).
In principle, both samples prepared from polymer 3 should be able to exchange PEG chains with the environment by breaking and forming the hydrazone bonds. However, in the presence of aniline this equilibrium was set much faster, as is clearly visible from Fig. 2a. The aniline containing sample reached its maximum fluorescence after 4.5 hours, while the non-catalyzed sample had not yet reached its equilibrium position after 30 hours. The negative control, prepared from polymer 6, does not contain a hydrazone bond and, therefore, was unable to exchange surface chains with its surroundings. Therefore, no increase in fluorescence was observed.
As these experiments were performed at neutral pH, it was expected that these polymeric vesicles kept their colloidal stability.18,19 To confirm this, we analyzed the size distribution of the polymeric vesicles at each time interval by dynamic light scattering (DLS). As shown in Fig. 2b and c no significant change in particle distribution was observed between 0 and 30 hours and, moreover, the derived count rate at a fixed measurement position appeared to be nearly equal for all data points, indicating identical concentration and size. This led us to conclude that indeed the surface of these polymersomes was in a dynamic equilibrium with its environment.
To take this concept one step further, we prepared polymeric vesicles of a combination of 3 and 4 in a ratio of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. These polymeric vesicles thus exhibited hydrazone bonds and were partially fluorescently labeled at both the inner and outer surface. As can be derived from the absence of fluorescence in the negative control in Fig. 2a (6 with aniline), free PEG is not able to cross the membrane of these polymeric vesicles on this timescale. Therefore PEG exchange is expected only to proceed on the polymersome outer surface, creating a vesicle with a different inner and outer functionalization. We repeated the aniline-catalyzed experiment by adding dansyl functionalized PEG (1) and aniline (12 equiv.) in solution to the rhodamine prefunctionalized polymersomes. Again, at each time point free PEG chains and aniline were separated from the polymersomes by size exclusion chromatography. The size and concentration of all samples were checked by DLS and found to be equal. Next, we analyzed the fluorescence intensity of both fluorophores separately in a 96 well plate reader (dansyl excitation 340 nm, emission 540 nm; rhodamine excitation 540 nm, emission 592 nm). Within five hours, the rhodamine intensity decreased to 0.5 (Fig. 3a), which is consistent with our hypothesis that only the outer membrane is susceptible to dynamic exchange. Furthermore, the emission of dansyl increased following an opposite trend compared to the decrease of rhodamine (Fig. 3b).
5. These polymeric vesicles thus exhibited hydrazone bonds and were partially fluorescently labeled at both the inner and outer surface. As can be derived from the absence of fluorescence in the negative control in Fig. 2a (6 with aniline), free PEG is not able to cross the membrane of these polymeric vesicles on this timescale. Therefore PEG exchange is expected only to proceed on the polymersome outer surface, creating a vesicle with a different inner and outer functionalization. We repeated the aniline-catalyzed experiment by adding dansyl functionalized PEG (1) and aniline (12 equiv.) in solution to the rhodamine prefunctionalized polymersomes. Again, at each time point free PEG chains and aniline were separated from the polymersomes by size exclusion chromatography. The size and concentration of all samples were checked by DLS and found to be equal. Next, we analyzed the fluorescence intensity of both fluorophores separately in a 96 well plate reader (dansyl excitation 340 nm, emission 540 nm; rhodamine excitation 540 nm, emission 592 nm). Within five hours, the rhodamine intensity decreased to 0.5 (Fig. 3a), which is consistent with our hypothesis that only the outer membrane is susceptible to dynamic exchange. Furthermore, the emission of dansyl increased following an opposite trend compared to the decrease of rhodamine (Fig. 3b).
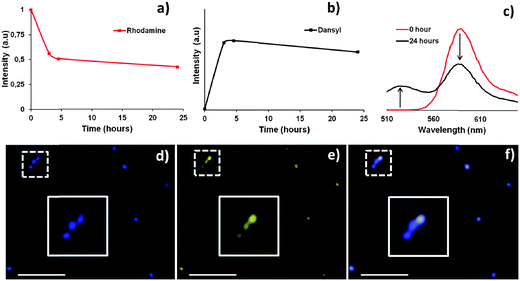 | ||
| Fig. 3 (a and b) Starting from rhodamine-functionalized polymersomes the outer rhodamine is replaced by a dansyl-functionalized surface. (c) Emission spectra of polymersomes before and after surface exchange of rhodamine-functionalized PEG chains by their fluorescein analogue. (d) CLSM visualization of rhodamine. (e) CLSM visualization of fluorescein on the same polymersomes. (f) CLSM overlay image of (d) and (e). The white bar represents 20 μm. | ||
To visualize the double functionalization of vesicles, we applied confocal laser scanning microscopy (CLSM). For this purpose we replaced dansyl-PEG for a fluorescein-PEG derivative (2) and repeated the experiment. As depicted in Fig. 3c (red) at time zero, after mixing and purification by SEC, the emission spectrum of the polymersomes only contained rhodamine emission (480 nm, for excitation of both fluorophores). However, when after 24 hours a sample was purified by size exclusion chromatography, the emission spectrum contained both rhodamine and fluorescein emissions. Furthermore, Fig. 3d–f show CLSM pictures obtained after 24 hours. Both fluorophores were excited at the same wavelength (476 nm) and selectively captured (fluorescein 500–530 nm (emission); rhodamine 580–610 (emission)). As can be seen from Fig. 3d–f, a good colocalization is observed for both fluorophores, indicating that both moieties are present on the same polymeric vesicle.
In the described experiments, it is not possible to directly visualize differences in inner and outer surface functionalization. This might be possible on giant unilamellar vesicles24 in combination with high resolution CLSM. However, the observations that the inner membrane cannot exchange, the original fluorescent probe is removed for 50%, and the fluorescent PEG added in solution is now attached to the polymersome surface clearly indicate that a different inner and outer membrane functionalization is obtained. Therefore we can conclude that the method as described herein allows for the substitution of the outer PEG surface of polymersomes for a new PEG surface containing a different functionalization.
In the preceding experiments we have shown that hydrazone-functionalized polymeric vesicles are able to exchange moieties with the surrounding solution. We next investigated whether this exchange would also take place between polymeric vesicles. For this purpose we synthesized a block copolymer analogue with a maleimide end group in which both blocks were linked via a hydrazone linkage (5). We formed polymersomes of a combination of 3 and 5 in a ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The maleimide functionalities were reacted with a cysteine residue connected to the cell penetrating tat peptide,25,26 resulting in tat-functional polymersomes.
2. The maleimide functionalities were reacted with a cysteine residue connected to the cell penetrating tat peptide,25,26 resulting in tat-functional polymersomes.
Rhodamine-functional polymersomes of 3 and 4 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were also formed as used in the previous experiments. Both types of polymersomes thus contained the hydrazone moiety between both blocks. Rhodamine polymersomes can be visualized by CLSM, but will not adhere to HeLa cells (Fig. S10†). Tat-polymersomes are invisible in CLSM (Fig. S9†), but are able to interact with the cells.7 If dynamic exchange between these vesicles takes place, the resulting set of polymersomes should be both fluorescent and have cell-adhesion capacity, as depicted schematically in Fig. 4a.
1) were also formed as used in the previous experiments. Both types of polymersomes thus contained the hydrazone moiety between both blocks. Rhodamine polymersomes can be visualized by CLSM, but will not adhere to HeLa cells (Fig. S10†). Tat-polymersomes are invisible in CLSM (Fig. S9†), but are able to interact with the cells.7 If dynamic exchange between these vesicles takes place, the resulting set of polymersomes should be both fluorescent and have cell-adhesion capacity, as depicted schematically in Fig. 4a.
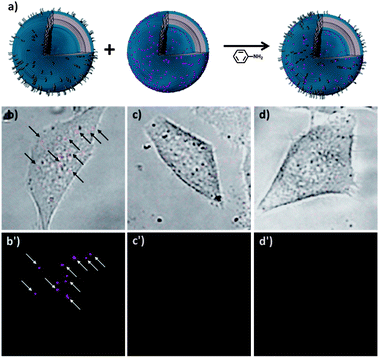 | ||
| Fig. 4 (a) Dynamic hydrazone exchange between vesicles functionalized with rhodamine and vesicles functionalized with cell penetrating tat-peptides will result in a single set of doubly functionalized vesicles. (b) After exchange polymersomes are both visible by CLSM (colored in red) and associated with HeLa cells. (c) Exchange in absence of tat vesicles will not yield vesicles that are both fluorescent and cell adhering. (d) If rhodamine labeled vesicles are prepared from block copolymers lacking the hydrazone moiety (6 and 7), the experiment will not yield vesicles that are both fluorescent and cell adhering. The top images of (b–d) show CLSM and bright field overlay images and the bottom CLSM images of 45 × 45 μm selections. The full images are shown in the ESI.† | ||
After formation of both the rhodamine- and the tat-polymersomes, they were mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and aniline was added. The exchange was allowed to proceed overnight, after which aniline and possible free PEG chains were removed by SEC. The sample was added to a HeLa cell culture and after washing the cells, CLSM indeed confirmed the presence of cell adhering, fluorescent polymersomes, as shown in Fig. 4b. This clearly shows that both the cell adhesion and fluorescent properties are combined in a single polymersome. To exclude false positive results, we included two negative controls in this study. When rhodamine polymersomes were reacted with aniline without the presence of tat-polymersomes, no polymersomes are visibly associated with HeLa cells (Fig. 4c). Secondly, if rhodamine-polymersomes were formed lacking the hydrazone moiety (6 and 7) and were reacted overnight in the presence of both aniline and tat polymersomes also no vesicles are visible in the HeLa cell culture (Fig. 4d).
1 ratio and aniline was added. The exchange was allowed to proceed overnight, after which aniline and possible free PEG chains were removed by SEC. The sample was added to a HeLa cell culture and after washing the cells, CLSM indeed confirmed the presence of cell adhering, fluorescent polymersomes, as shown in Fig. 4b. This clearly shows that both the cell adhesion and fluorescent properties are combined in a single polymersome. To exclude false positive results, we included two negative controls in this study. When rhodamine polymersomes were reacted with aniline without the presence of tat-polymersomes, no polymersomes are visibly associated with HeLa cells (Fig. 4c). Secondly, if rhodamine-polymersomes were formed lacking the hydrazone moiety (6 and 7) and were reacted overnight in the presence of both aniline and tat polymersomes also no vesicles are visible in the HeLa cell culture (Fig. 4d).
Conclusions
In conclusion, we have introduced a new concept to functionalize polymeric vesicles in a dynamic fashion. We have shown that polymersomes formed from a hydrazone-coupled block copolymer exchange their surface functionality, both with dissolved species and with other vesicles via the hydrazone equilibrium. Via this method, polymeric vesicles can be conveniently formed with different inner and outer functionalities. Furthermore, multiple functional surfaces can be obtained via one single exchange experiment by mixing the appropriate sets of polymersomes.Acknowledgements
This study/work was performed within the framework of the Dutch Top Institute Pharma project #T5-105. The authors thank Prof. Dr Kyoung Taek Kim for pointing to the aniline catalyzed hydrazone equilibrium and Hans Adams for the Tat peptide synthesis.Notes and references
- B. M. Discher, Y. Y. Won, D. S. Ege, J. C. M. Lee, F. S. Bates, D. E. Discher and D. A. Hammer, Science, 1999, 284, 1143–1146 CrossRef CAS.
- Z. Q. Pang, W. Lu, H. L. Gao, K. L. Hu, J. Chen, C. L. Zhang, X. L. Gao, X. G. Jiang and C. Q. Zhu, J. Controlled Release, 2008, 128, 120–127 CrossRef CAS.
- F. Ahmed, R. I. Pakunlu, A. Brannan, F. Bates, T. Minko and D. E. Discher, J. Controlled Release, 2006, 116, 150–158 CrossRef CAS.
- Z. L. Cheng and A. Tsourkas, Langmuir, 2008, 24, 8169–8173 CrossRef CAS.
- D. A. Christian, O. B. Garbuzenko, T. Minko and D. E. Discher, Macromol. Rapid Commun., 2010, 31, 135–141 CAS.
- S. Egli, H. Schlaad, N. Bruns and W. Meier, Polymers, 2011, 3, 252–280 CrossRef CAS.
- N. A. Christian, M. C. Milone, S. S. Ranka, G. Z. Li, P. R. Frail, K. P. Davis, F. S. Bates, M. J. Therien, P. P. Ghoroghchian, C. H. June and D. A. Hammer, Bioconjugate Chem., 2007, 18, 31–40 CrossRef CAS.
- F. H. Meng, G. H. M. Engbers and J. Feijen, J. Controlled Release, 2005, 101, 187–198 CrossRef CAS.
- R. Nehring, C. G. Palivan, S. Moreno-Flores, A. Mantion, P. Tanner, J. L. Toca-Herrera, A. Thunemann and W. Meier, Soft Matter, 2010, 6, 2815–2824 RSC.
- J. M. Lehn and A. V. Eliseev, Science, 2001, 291, 2331–2332 CrossRef CAS.
- C. B. Minkenberg, F. Li, P. van Rijn, L. Florusse, J. Boekhoven, M. C. A. Stuart, G. J. M. Koper, R. Eelkema and J. H. van Esch, Angew. Chem., Int. Ed., 2011, 50, 3421–3424 CrossRef CAS.
- S. Cerritelli, D. Velluto and J. A. Hubbell, Biomacromolecules, 2007, 8, 1966–1972 CrossRef CAS.
- B. K. Sourkohi, A. Cunningham, Q. Zhang and J. K. Oh, Biomacromolecules, 2011, 12, 3819–3825 CrossRef.
- B. K. Sourkohi, R. Schmidt and J. K. Oh, Macromol. Rapid Commun., 2011, 32, 1652–1657 CrossRef.
- S. Egli, M. G. Nussbaumer, V. Balasubramanian, M. Chami, N. Bruns, C. Palivan and W. Meier, J. Am. Chem. Soc., 2011, 133, 4476–4483 CrossRef CAS.
- J. H. Ryu, R. T. Chacko, S. Jiwpanich, S. Bickerton, R. P. Babu and S. Thayumanavan, J. Am. Chem. Soc., 2010, 132, 17227–17235 CrossRef CAS.
- Y. Bae, S. Fukushima, A. Harada and K. Kataoka, Angew. Chem., Int. Ed., 2003, 42, 4640–4643 CrossRef CAS.
- R. P. Brinkhuis, T. R. Visser, F. P. J. T. Rutjes and J. C. M. van Hest, Polym. Chem., 2011, 2, 550–552 RSC.
- L. He, Y. Jiang, C. L. Tu, G. L. Li, B. S. Zhu, C. Y. Jin, Q. Zhu, D. Y. Yan and X. Y. Zhu, Chem. Commun., 2010, 46, 7569–7571 RSC.
- E. H. Cordes and W. P. Jencks, J. Am. Chem. Soc., 1962, 84, 826 CrossRef CAS.
- A. Dirksen, S. Dirksen, T. M. Hackeng and P. E. Dawson, J. Am. Chem. Soc., 2006, 128, 15602–15603 CrossRef CAS.
- A. Dirksen, S. Yegneswaran and P. E. Dawson, Angew. Chem., Int. Ed., 2010, 49, 2023–2027 CrossRef CAS.
- A. Dirksen and P. E. Dawson, Bioconjugate Chem., 2008, 19, 2543–2548 CrossRef CAS.
- Y. F. Zhou and D. Y. Yan, J. Am. Chem. Soc., 2005, 127, 10468–10469 CrossRef CAS.
- M. Green and P. M. Loewenstein, Cell, 1988, 55, 1179–1188 CrossRef CAS.
- A. D. Frankel and C. O. Pabo, Cell, 1988, 55, 1189–1193 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterisation. See DOI: 10.1039/c2py20789c |
| This journal is © The Royal Society of Chemistry 2013 |
