Ultrafast electronic deactivation dynamics of the inosine dimer – a model case for H-bonded purine bases
Abstract
The structural properties and ultrafast electronic deactivation dynamics of the
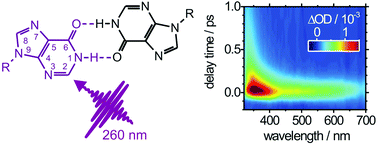
- This article is part of the themed collection: Interaction of UV radiation with DNA

 Please wait while we load your content...
Please wait while we load your content...