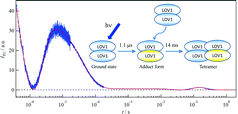
The photochemical reaction of the LOV1 (light-oxygen-voltage 1) domain of phototropin 1 from Arabidopsis thaliana was investigated by the time-resolved transient grating method. As with other LOV domains, an absorption spectral change associated with an adduct formation between its chromophore (flavin mononucleotide) and a cysteine residue was observed with a time constant of 1.1 μs. After this reaction, a significant diffusion coefficient (D) change (D of the reactant = 8.2 × 10−11 m2 s−1, and D of the photoproduct = 6.4 × 10−11 m2 s−1) was observed with a time constant of 14 ms at a protein concentration of 270 μM. From the D value of the ground state and the peak position in size exclusion chromatography, we have confirmed that the phot1LOV1 domain exists as a dimer in the dark. The D-value and the concentration dependence of the rate indicated that the phot1LOV1 domain associates to form a tetramer (dimerization of the dimer) upon photoexcitation. We also found that the chromophore is released from the binding pocket of the LOV domain when it absorbs two photons within a pulse duration, which occurs in addition to the normal photocycle reaction. On the basis of these results, we discuss the molecular mechanism of the light dependent role of the phot1LOV1 domain.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...