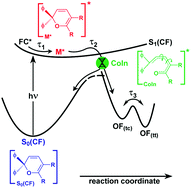
In this paper we investigate the photochromic ring-opening reaction of 2,2-diphenyl-5,6-benzo(2H)chromene. In particular, we study the uncertainties and contradictions in various published reaction models using a combination of transient absorption and fluorescence spectroscopy with femtosecond time resolution. We propose a simplified reaction scheme which is in good agreement with theoretical studies. Here, photoexcitation populates a Franck–Condon state, whose fast vibrational wave packet motion, vibrational relaxation, bond-alternation and/or solvent rearrangement processes occur on the sub-picosecond timescale. Our data suggest that the resulting excited state minimum with picosecond lifetime still features structural characteristics of the closed form. Subsequently, the ring-opened photoproducts are formed in a concerted step from the excited state. The velocity of the photoreaction hence only depends on the time that the molecule needs to reach the transition region between the ground and excited states where the crucial bond breakage occurs.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...