In celebration of the 60th birthday of Professor Andrew D. Hamilton FRS†
Sam Thompsona, Andrew J. Wilsonb and Alan R. Battersbyc
aChemistry Research Laboratory, University of Oxford, Mansfield Road, Oxford OX1 3TA, UK. E-mail: sam.thompson@chem.ox.ac.uk
bSchool of Chemistry and Astbury Centre for Structural Molecular Biology, University of Leeds, Woodhouse Lane, Leeds LS2 9JT, UK. E-mail: a.j.wilson@leeds.ac.uk
cUniversity Chemical Laboratory, Cambridge University, Lensfield Road, Cambridge CB2 1EW, UK. E-mail: arb1005@cam.ac.uk
Abstract
An on-line collection of articles celebrating the 60th birthday of Professor Andrew D. Hamilton FRS has been published featuring contributions from students and colleagues past and present. This article hopes to provide an insight into the rise of a star in molecular recognition, ground breaking discoveries, and on a more light-hearted note, some fond reminiscences of research in Cambridge, Princeton, Pittsburgh, Yale and Oxford.
 Sam Thompson | Sam Thompson received an MChem from Exeter College, Oxford (2004) spending his final year with Ben Davis in the Dyson Perrins Laboratory. He moved to St Edmund's College, Cambridge for a PhD (2008) with Martin Smith working on cascade routes to polycyclic alkaloids. Returning to Oxford he took up a position as Junior Research Fellow at Pembroke College, Oxford and postdoctoral fellowship with Andrew Hamilton. He now holds a second Junior Research Fellowship at Lady Margaret Hall. His interests lie in the use of organic synthesis as a tool to probe problems in biology and chemistry, and application of this to unmet challenges in medicine. |
 Andrew J. Wilson | Andrew Wilson is currently Professor of Organic Chemistry at the University of Leeds and Deputy Director of the Astbury Centre for Structural Molecular Biology. His research is concerned with (a) disrupting biological processes, specifically protein–protein interactions, (b) developing fundamental approaches and building blocks for self-assembly and (c) mechanistic studies of self-assembly. |
 Alan R. Battersby | Sir Alan Battersby was born in 1925 and studied at the Universities of Manchester and St Andrews (PhD 1949). He gained a Commonwealth Fund Fellowship (1950–52) for study in the United States at the Rockefeller Institute and the University of Illinois. He then joined the faculty at the University of Bristol and initiated his research on biosynthesis. In 1962, he accepted a Chair at the University of Liverpool then, in 1969, he moved to a Chair at Cambridge, finally holding the Cambridge 1702 Chair. He became Emeritus Professor in 1992 and continued both experimental research and his writing. His research interests have been broadly concerned with the chemistry of living systems. Isolation work, structure determination, synthesis, isotopic labeling and spectroscopy have been combined in these studies synergistically with enzymology and molecular biology. Three main themes can be picked out: (a) biosynthesis of alkaloids; (b) stereochemistry of enzymic reactions; (c) biosynthesis of porphyrins and vitamin B12. Sir Alan has received various awards including the Corday–Morgan, Tilden, Hugo Muller, Flintoff, Pedler and Longstaff Medals and the Award for the Chemistry of Natural Products all from the Royal Society of Chemistry. He was awarded the Davy, Royal and Copley Medals of the Royal Society, the Paul Karrer Medal and the August Wilhelm von Hofmann Award. The Roger Adams Medal came in 1983 followed by the Havinga Medal and the Antonio Feltrinelli International Prize. Recent notable awards are the Adolf Windaus Medal, the Wolf Prize, the August Wilhelm von Hofmann Memorial Medal and the Tetrahedron Prize for Creativity in Organic Chemistry. He was knighted in 1992. |
Professor Andy Hamilton FRS (Fig. 1) is known throughout the world for his research in the areas of supramolecular-, bioorganic-, and medicinal chemistry. In terms of publications in these areas there are few within science that would not instantly recognise a “Hamilton” without having to check the list of authors! Recently Andy turned 60 and a number of his current and former co-workers felt a reunion was in order. Now, to a certain extent what went on in Oxford during that event will have to stay in Oxford (see below), but it is fair to say that this extended “group” meeting clearly charted the evolution of (a) a research programme geared towards emulating nature and reproducing molecular recognition using synthetic designed molecules; (b) the mentoring of a significant number of co-workers who now deliver their own academic and industrial research, and teach, promote or administer science. In this latter aspect many of us have benefited from Andy's enthusiasm, inspiration and positivity over the last 30 years. In a similar manner, this special issue recognises Andy's contribution to science more formally and his stimulation of the wider community. Many of you reading this editorial will have contributed articles and it is fair to say the warmth felt for one of the leaders of the community is easy to see. We hope the following pages will draw together some common themes!
 | ||
| Fig. 1 Professor Hamilton pictured in Oxford against a backdrop of the Radcliffe Camera and the University Church of St Mary the Virgin. | ||
Early years
Born in November 1952, Andy Hamilton left his native Surrey to read chemistry at the University of Exeter. Always eager to travel, an MSc at the University of British Columbia, Vancouver, with David Dolphin followed. Working with bile-pigments1,2 initiated a lifelong interest in iron-containing proteins and his move to St John's College, Cambridge in 1976 to join Alan Battersby's team working on porphyrins and corrins (B12) sparked his interest in what was to become the theme of his research career; many examples follow in this article. Andy grasped the chance to take on the project of mimicking the binding of oxygen and carbon monoxide at the iron centre of haemoglobin. This involved building bridges across both faces of a porphyrin macrocycle so that the ligation of the metal centre and the space available on the other face could be varied and controlled. Andy was a courageous and effective pioneer in developing the synthetic routes and methodologies that were required and he carried these skills when he worked with another team member to model the porphyrin centre of cytochrome c. The Cambridge group functioned in that way; it was tightly knit, cooperative and friendly. Andy's personality matched it perfectly. Even then, his ability to manage several different activities at the same time was evident. All this vigorous research effort was combined seamlessly with his enthusiasm for playing rugby. On completing an outstanding PhD (Fig. 2a),3–6 Andy moved to the Université Louis Pasteur in Strasbourg as a NATO-Royal Society European Exchange Fellow. A keen interest in bioorganic chemistry and supramolecular mimicry grew working alongside John Sessler and Jean-Marie Lehn. The latter would go on to win the Nobel Prize a few years later.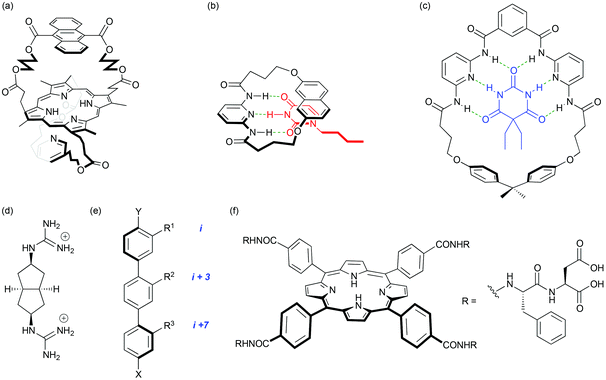 | ||
| Fig. 2 (a) A porphyrin with an anthracene bridge across one face and a pyridine bridge across the other; this molecule binds Fe(II) and mimics the structural features and properties of natural oxygen-carrying systems; (b) nucleotide base recognition with a molecular hinge; (c) a macrocyclic tetra-amide barbiturate binder. Seminal work in the development of proteomimetics: (d) selective binding of aspartate pairs in helical peptides with a guanidinium-based receptor; (e) the terphenyl – an early α-helix mimetic; (f) a tetraphenylporphyrin binder of cytochrome c illustrating the importance of both hydrophilic and hydrophobic interactions. | ||
Independent career
Appointed as an Assistant Professor at Princeton in 1981, seven years of research looked at the vancomycin problem and the development of greatly simplified synthetic scaffolds able to bind selectively to small organic molecules such as peptides. At this stage Andy's vision was clear; molecular recognition in aqueous solvent was considered a long way off. The aims of the group became firmly focussed on the use of synthetic design for the understanding, mimicry and potential disruption of biological processes. Joining Pittsburgh as an Associate Professor in 1988 and going on to become Professor (1992) and Departmental Chair (1994), the research interests of the group expanded into investigations of neutral guests through hydrogen-bonding. Major papers followed on the recognition of nucleotides7 using a molecular hinge and the famous barbiturate receptor8 used as an acyclic building block and affectionately referred to as the “Hamilton Wedge” (Fig. 2b, c).It is worth noting Prof. Hamilton to this day delights in reminding those of us who were yet to become researchers that ChemDraw was not readily available at the time! Attempts to get back to recognition events in more challenging solvents quickly followed. This included receptors for dicarboxylates9 and some of the first attempts to use non-covalent interactions to direct conformational preference.10,11 As the stay in Pittsburgh progressed, some early thoughts on (dynamic) combinatorial receptor design and protein recognition were to emerge (Fig. 2d).12–14 It was during the Pittsburgh years that Andy also formed his most productive collaboration, with cancer biologist Saïd Sebti, developing synthetic inhibitors of the farnesyl- and geranyl-transferases.15
A new chapter of Andy's career opened with a move to Yale as The Irénée DuPont Professor of Chemistry in 1997 and with it an increased role in university administration was to follow. Those in the group at the time talk affectionately of the drive over the mountains! In the course of eleven years at Yale he would go on to become Professor at the Department of Molecular Biophysics, Departmental Chair, Deputy Provost for Science and Technology, The Benjamin Silliman Professor of Chemistry and in 2004 Senior Provost. Most people would struggle to manage these responsibilities without the inevitable fall in scientific productivity. Somehow the opposite proved to be the case, with the period at Yale seeing expansion into the fields of protein surface recognition and further work on self-assembly of small molecular gelators, punctuated by the publication of over 260 manuscripts. This period saw ground breaking work on the first true mimics of protein secondary structure16,17 and an entirely new use of calixarenes18,19 and subsequently porphyrins20 as scaffolds for recognition of proteins (Fig. 2e, f).
Following his inexorable rise through the ranks at Yale, it was perhaps unsurprising that in June 2008 The University of Oxford announced Hamilton's nomination as Vice-Chancellor, and two weeks later, his election to the seven year post beginning October 2009.
A group of two postdocs and two graduate students from Yale took on the challenge of transplanting the lab from New Haven and re-establishing it in Oxford. This proved to be an eventful move, with one of the team briefly confined to the brig aboard the Queen Mary II during the transatlantic crossing after a misunderstanding at The Captain's table. Fortunately the Americans quickly settled into Oxford life, with a lively cultural exchange operating in both directions. Thanksgiving is now firmly established on the group's social calendar.
The group has continued to pioneer synthetic approaches to the inhibition or stabilisation of protein–protein interactions21–24 (Fig. 3) and has formed collaborations in Oxford with medicine, chemistry and structural biology. A growing awareness of the importance, and potential therapeutic benefits of understanding and controlling the recognition events involved in post-translational modification has provided a renewed impetus for the design and synthesis of peptidomimetics. There is a particular focus on the use of self-assembled architectures for the recognition and reproduction of higher-order protein elements and the surfaces they present (Fig. 4).25–27
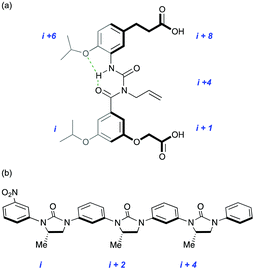 | ||
| Fig. 3 New generations of protein secondary structure mimetic: (a) amphiphilic α-helix; (b) β-strand. | ||
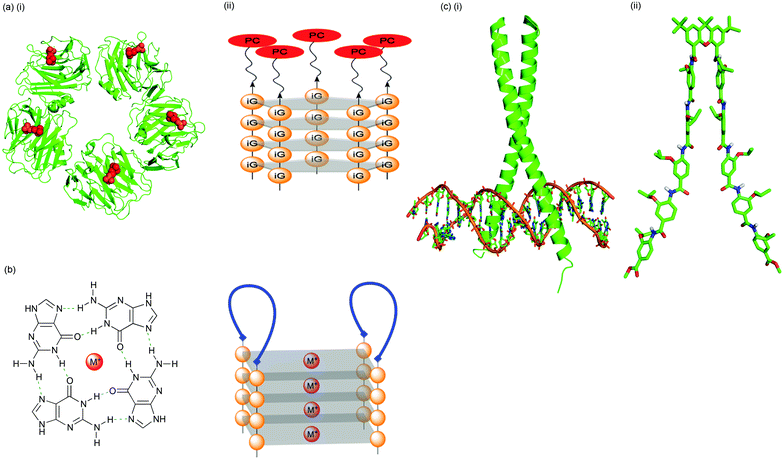 | ||
| Fig. 4 Strategies for exploring protein surface recognition: (a) (i) phosphocholine (PC, red) binding of a pentameric recognition protein (C-reactive protein, CRP, green; pdb 1B09); (ii) PC assembled on a DNA, deoxyisoguanosine pentaplex scaffold. CRP binding affinity can be tuned by the choice of metal ion used for promotion of self-assembly; (b) a non-covalent strategy for template-assembled protein design. A peptide is conjugated with two oligoguanosine strands and the conjugates self-assembled in the presence of metal ions. G-quadruplex formation directs two peptide strands (blue) to assemble on one face of the scaffold and form an adjacent two-loop surface; (c) the mimicry of supersecondary protein structure (i) the GCN4 leucine zipper binding DNA (pdb 1YSA); (ii) a bis-pentabenzamide peptidomimetic as a potential synthetic transcription factor. | ||
The growing field of molecular scale devices has seen a number of contributions from the lab in the form of diphenylacetylene-based single-molecule switches that can reversibly, and selectively, sense the presence of a cation or anion.28–30 These systems have potential uses in storage, display, sensing and medicine (Fig. 5).
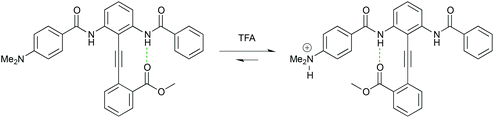 | ||
| Fig. 5 The conformational equilibrium of a pH-dependent switch based on an intramolecularly H-bonded diphenylacetylene. Protonation of the electron-donating dimethylamino group converts it into an electron-withdrawing dimethylammonium cation with a concomitant switch in conformation. | ||
Although all of these interests will continue to be represented, it is perhaps the defining quality of Hamilton's research that he continually reinvents himself and develops new fields. As a junior co-worker there is always trepidation when Andy arrives, starts to doodle on the back of a notebook or fumehood sash and throws out an idea for a new research project for which there is no precedent or prior knowledge in the lab, normally ending with the line: I have a meeting to go to … but you can work out the details!
Awards
Andy's scientific and administrative work has been recognised by half a dozen honorary degrees, more than twenty distinguished lectureships and numerous honours and prizes. Some of the highlights are the Arthur C. Cope Scholar Award of the American Chemical Society (1999), membership of the American Association for the Advancement of Science, and elections as Fellow of the Royal Society of Chemistry, and the Royal Society (all 2004). In 2010 he was elected to the American Academy of Arts and Sciences and in 2011 was the winner of The International Izatt-Christiansen Award for Macrocyclic Chemistry.Symposium
June 2012 saw a symposium held in Oxford in honour of Andy's birthday, attended by 60 members of his current and former group. There were short talks from many of those who have contributed articles to this special edition. We heard about CGRP receptor antagonists from the first Hamilton Group graduate Gene Dubowchik, drugging oncogenic STAT3 protein function (Patrick Gunning), protein labelling and engineering (Itaru Hamachi) and heard a career retrospective from Andy, generously titled ‘In denial: how working with a fabulous research group keeps you young’ (Fig. 6). | ||
| Fig. 6 A gathering of current and former group members in Oxford during the summer of 2012. | ||
We are delighted that so many friends and colleagues have contributed articles to this special edition and that Andy's influence and inspiration is evident in much of this work.
References
- A. Hamilton and D. Dolphin, Heterocycles, 1977, 7, 817–829 CrossRef CAS.
- K. Mailer, R. Poulson, D. Dolphin and A. Hamilton, Biochem. Biophys. Res. Commun., 1980, 96, 777–784 CrossRef CAS.
- A. R. Battersby, A. D. Hamilton, E. McDonald, L. Mombelli and O.-H. Wong, J. Chem. Soc., Perkin Trans. 1, 1980, 1283–1289 RSC.
- W. B. Cruse, O. Kennard, G. M. Sheldrick, A. D. Hamilton, S. G. Hartley and A. R. Battersby, J. Chem. Soc., Chem. Commun., 1980, 700–701 RSC.
- A. R. Battersby and A. D. Hamilton, J. Chem. Soc., Chem. Commun., 1980, 117–119 RSC.
- A. R. Battersby, W. Howson and A. D. Hamilton, J. Chem. Soc., Chem. Commun., 1982, 1266–1268 RSC.
- A. D. Hamilton and D. Van Engen, J. Am. Chem. Soc., 1987, 109, 5035–5036 CrossRef CAS.
- S. K. Chang and A. D. Hamilton, J. Am. Chem. Soc., 1988, 110, 1318–1319 CrossRef CAS.
- F. Garcia-Tellado, S. Goswami, S. K. Chang, S. J. Geib and A. D. Hamilton, J. Am. Chem. Soc., 1990, 112, 7393–7394 CrossRef CAS.
- S. J. Geib, C. Vicent, E. Fan and A. D. Hamilton, Angew. Chem., Int. Ed. Engl., 1993, 32, 119–121 CrossRef.
- Y. Hamuro, S. J. Geib and A. D. Hamilton, Angew. Chem., Int. Ed. Engl., 1994, 33, 446–448 CrossRef.
- J. S. Albert, M. S. Goodman and A. D. Hamilton, J. Am. Chem. Soc., 1995, 117, 1143–1144 CrossRef CAS.
- M. S. Goodman, A. D. Hamilton and J. Weiss, J. Am. Chem. Soc., 1995, 117, 8447–8455 CrossRef CAS.
- M. S. Goodman, V. Jubian, B. Linton and A. D. Hamilton, J. Am. Chem. Soc., 1995, 117, 11610–11611 CrossRef CAS.
- E. C. Lerner, Y. Qian, M. A. Blaskovich, R. D. Fossum, A. Vogt, J. Sun, A. D. Cox, C. J. Der, A. D. Hamilton and S. M. Sebti, J. Biol. Chem., 1995, 270, 26802–26806 CrossRef CAS.
- B. P. Orner, J. T. Ernst and A. D. Hamilton, J. Am. Chem. Soc., 2001, 123, 5382–5383 CrossRef CAS.
- J. T. Ernst, J. Becerril, H. S. Park, H. Yin and A. D. Hamilton, Angew. Chem., Int. Ed., 2003, 42, 535–539 CrossRef CAS.
- Y. Hamuro, M. C. Calama, H. S. Park and A. D. Hamilton, Angew. Chem., Int. Ed. Engl., 1997, 36, 2680–2683 CrossRef CAS.
- M. A. Blaskovich, Q. Lin, F. L. Delarue, J. Sun, H. S. Park, D. Coppola, A. D. Hamilton and S. M. Sebti, Nat. Biotechnol., 2000, 18, 1065–1070 CrossRef CAS.
- R. K. Jain and A. D. Hamilton, Org. Lett., 2000, 2, 1721–1723 CrossRef CAS.
- S. Thompson, R. Vallinayagam, M. J. Adler, R. T. W. Scott and A. D. Hamilton, Tetrahedron, 2012, 68, 4501–4505 CrossRef CAS.
- S. Thompson and A. D. Hamilton, Org. Biomol. Chem., 2012, 10, 5780–5782 CAS.
- M. J. Adler, R. T. W. Scott and A. D. Hamilton, Chem.–Eur. J., 2012, 18, 12974–12977 CrossRef CAS.
- C. L. Sutherell, S. Thompson, R. T. W. Scott and A. D. Hamilton, Chem. Commun., 2012, 48, 9834–9836 RSC.
- B. A. Rosenzweig, N. T. Ross, M. J. Adler and A. D. Hamilton, J. Am. Chem. Soc., 2010, 132, 6749–6754 CrossRef CAS.
- P. S. Ghosh and A. D. Hamilton, J. Am. Chem. Soc., 2012, 134, 13208–13211 CrossRef CAS.
- O. V. Kulikov, S. Thompson, H. Xu, C. D. Incarvito, R. T. W. Scott, I. Saraogi, L. Nevola and A. D. Hamilton, Eur. J. Org. Chem., 2013, 3433–3445 CrossRef CAS.
- I. M. Jones and A. D. Hamilton, Org. Lett., 2010, 12, 3651–3653 CrossRef CAS.
- I. M. Jones and A. D. Hamilton, Angew. Chem., Int. Ed., 2011, 50, 4597–4600 CrossRef CAS.
- I. M. Jones, H. Lingard and A. D. Hamilton, Angew. Chem., Int. Ed., 2011, 50, 12569–12571 CrossRef CAS.
Footnote |
| † Part of the themed issue in celebration of Professor Andrew D. Hamilton's 60th birthday. |
| This journal is © The Royal Society of Chemistry 2013 |
