Rajeev R. Jha, Rakesh K. Saunthwal and Akhilesh K. Verma
Org. Biomol. Chem., 2014,12, 552-556
DOI:
10.1039/C3OB42035C,
Communication
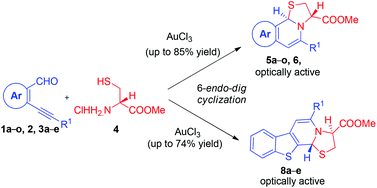
An operationally simple approach for the stereoselective tandem synthesis of novel thiazolo fused naphthyridines 5a–o and thienopyridines 8a–e by the reaction of o-alkynylaldehydes with L-cystine methyl ester hydrochloride via Au(III)-catalyzed regioselective 6-endo-dig ring closure under mild reaction conditions is described. It is noteworthy that alkynes bearing an alkyl and a strong electron-withdrawing nitro group successfully afforded the desired products in good yields.