Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stereoselective preparation of lipidated carboxymethyl-proline/pipecolic acid derivatives via coupling of engineered crotonases with an alkylmalonyl-CoA synthetase†
Refaat B.
Hamed
*ab,
Luc
Henry
a,
J. Ruben
Gomez-Castellanos
a,
Amina
Asghar
a,
Jürgen
Brem
a,
Timothy D. W.
Claridge
a and
Christopher J.
Schofield
*a
aDepartment of Chemistry, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA, UK. E-mail: christopher.schofield@chem.ox.ac.uk; Fax: +44 (0)1865275674; Tel: +44 (0)1865275625
bDepartment of Pharmacognosy, Faculty of Pharmacy, Assiut University, Assiut, 71526, Egypt, (on leave). E-mail: refaat.hamed@outlook.com
First published on 3rd October 2013
Abstract
The trisubstituted enolate- and C–C bond-forming capacities of engineered carboxymethylproline synthases CMPSs are coupled with the malonyl-CoA synthetase MatB to enable stereoselective preparation of 5- and 6-membered N-heterocycles functionalised with alkyl-substituted carboxymethyl side chains, starting from achiral alkyl-substituted malonic acids and L-amino acid semialdehydes. The results illustrate the biocatalytic utility of crotonases in tandem enzyme-catalysed reactions for stereoselective synthesis.
Introduction
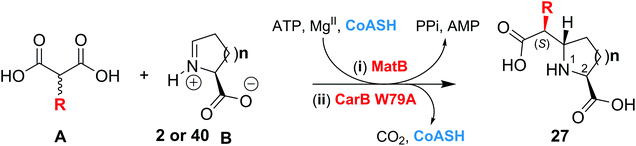
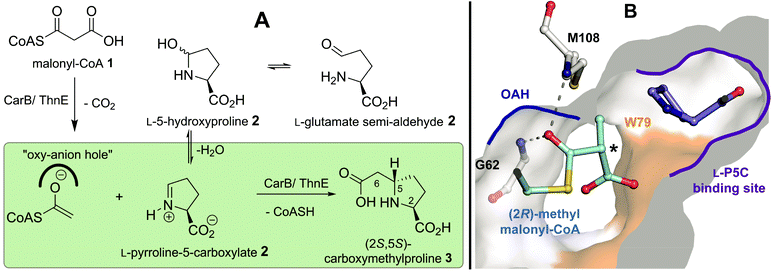
Saturated 5- and 6-membered N-heterocycles are important constituents of many natural products and pharmaceuticals (e.g. oxaceprol, captopril and tacrolimus) and are used as catalysts1 in asymmetric synthesis (e.g. proline derivatives). The development of “asymmetric” biocatalytic routes for the versatile production of functionalised N-heterocycles is thus of interest.The carboxymethylproline synthases (CMPSs, CarB from Pectobacterium carotovorum2–5 and ThnE from Streptomyces cattleya6–8) catalyse an N-heterocycle ring-forming step in carbapenem biosynthesis and are members of the crotonase superfamily.9,10 CarB and ThnE catalyse reaction of malonyl-CoA 1 with an equilibrating mixture of L-glutamate semialdehyde/5-hydroxyproline/pyrroline-5-carboxylate (collectively L-GHP, 2) to give (2S,5S)-carboxymethylproline (t-CMP) 3 (Fig. 1A).
 | ||
| Fig. 1 Engineering carboxymethylproline synthases to accept malonyl-CoA 1 analogues with bulky side chains. (A) CarB and ThnE catalysed synthesis of (2S,5S)-carboxymethylproline 3;7,10 (B) A view from a CarB structure2 with (2R)-methylmalonyl-CoA 4 and L-pyrroline-5-carboxylate 2 (L-P5C) modeled in the active site. The Trp79 residue, the surface of which is shown in orange, is part of a hydrophobic face in the active site; substitution of Trp79 for Phe- or Ala-residues increases the capacity of CarB to accommodate bulkier substituents at the malonyl-CoA C-2 position (asterisked). | ||
The mechanisms of catalysis of most crotonases, including CarB and ThnE, are proposed to proceed via enolate intermediates, usually generated by decarboxylation of (a derivative of) 1. The enolate intermediates are stabilised by a conserved oxyanion hole (OAH), which is formed in the case of CarB and ThnE by residues Gly62CarB/Gly107ThnE and Met108CarB/Val153ThnE (Fig. 1). C–C bond formation can then proceed via reaction of the enolate intermediate with the imine form of 2 to give the t-CMP-CoA thioester, which is then hydrolysed to give 3 and coenzyme A (CoASH) (Fig. 1A).4,5
Despite its central importance in asymmetric synthesis, the stereoselective alkylation of enolates has been relatively under-explored for biocatalytic C–C bond formation with notable exceptions including the use of engineered aldolases and catalytic antibodies.11–14 We have reported that (engineered) CMPSs accept L-GHP analogues to give 6- and 7-membered carboxymethyl-N-heterocycles.15 CMPSs also accept methylated derivatives of L-GHP to give 5-carboxymethylproline derivatives functionalised at C-2, C-3, C-4, or C-5 of the proline ring, including products with a quaternary center (at C-2 or C-5) in a stereoselective fashion.16 Active site CMPS variants enabled the stereoselective alkylation of enolates generated from methylmalonyl-CoA 4 and ethylmalonyl-CoA 5.17 These findings stimulated us to investigate other C-2 alkylmalonyl-CoA derivatives as CMPS substrates, and whether the stereoselectivity of CMPS catalysis could be improved.
We report that CMPS catalysis can be coupled to that of malonyl-CoA synthetase (MatB) or crotonyl-CoA carboxylase reductase (Ccr) for the stereoselective preparation of functionalised prolines and pipecolic acids. The results illustrate the utility of coupling crotonases with other enzymes for stereoselective synthesis. With appropriate optimization, the reactions may be useful in cell-based contexts for the preparation of functionalised heterocycles for use in pharmaceutical research.
Results and discussion
Incubation of acryloyl-CoA derivatives, L-GHP, crotonyl-CoA carboxylase reductase and CMPS variants
When C-2 epimeric 4 is incubated with CarB and L-GHP 2, a ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the (6R)- and (6S)-epimers of 6-methyl-t-CMP 6 is produced (Fig. 2A).4,5,17 Similarly, incubation of C-2 epimeric 5 with CarB and L-GHP gave an ∼65
1 mixture of the (6R)- and (6S)-epimers of 6-methyl-t-CMP 6 is produced (Fig. 2A).4,5,17 Similarly, incubation of C-2 epimeric 5 with CarB and L-GHP gave an ∼65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 mixture of (6R)- and (6S)-epimers of 6-ethyl-t-CMP 7.
35 mixture of (6R)- and (6S)-epimers of 6-ethyl-t-CMP 7.
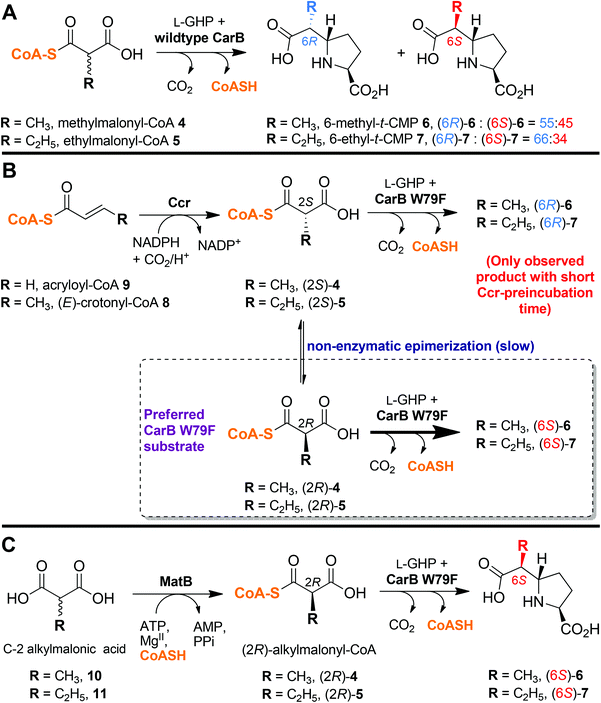 | ||
Fig. 2 Coupling carboxymethylproline synthases with alkylmalonyl-CoA forming enzymes. (A) CarB catalyses formation of a ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of C-6 epimers of 6/7 on incubation with C-2 epimeric 4/5; (B) Coupling of crotonyl-CoA carboxylase reductase (Ccr)-catalysed formation of (2S)-4/518,19 with CMPS to give (6R)-6 or -7.17 Note that (6S)-6 or -7 are only observed when the Ccr incubation time is sufficiently long prior to CMPS addition; (C) Coupling of malonyl-CoA synthetase (MatB)-catalysed formation of (2R)-alkylmalonyl-CoA20 with CarB W79F for formation of (6S)-6 or -7. 1 mixture of C-6 epimers of 6/7 on incubation with C-2 epimeric 4/5; (B) Coupling of crotonyl-CoA carboxylase reductase (Ccr)-catalysed formation of (2S)-4/518,19 with CMPS to give (6R)-6 or -7.17 Note that (6S)-6 or -7 are only observed when the Ccr incubation time is sufficiently long prior to CMPS addition; (C) Coupling of malonyl-CoA synthetase (MatB)-catalysed formation of (2R)-alkylmalonyl-CoA20 with CarB W79F for formation of (6S)-6 or -7. | ||
We have developed CMPS variants that selectively convert (2S)-4/5 or (2R)-4/5 to (6R)-6/7 and (6S)-6/7, respectively.17 The ratio of (6R)-![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (6S)-6/7 epimers observed depends on factors including the ‘intrinsic’ selectivity of the CMPS variant, the overall extent of reaction (as the concentration of a specific C-2 epimer of 4/5 is diminished, the rate of conversion of the other is enhanced) and the rate of non-enzymatic interconversion between the (2S)- and (2R)-4/5 epimers (the faster the equilibration, the more CMPS-variant catalysed conversion of the favoured CMPS substrate is observed).17
(6S)-6/7 epimers observed depends on factors including the ‘intrinsic’ selectivity of the CMPS variant, the overall extent of reaction (as the concentration of a specific C-2 epimer of 4/5 is diminished, the rate of conversion of the other is enhanced) and the rate of non-enzymatic interconversion between the (2S)- and (2R)-4/5 epimers (the faster the equilibration, the more CMPS-variant catalysed conversion of the favoured CMPS substrate is observed).17
(2S)-5 can be produced, as a single (>90%) (2S)-epimer by reaction of crotonyl-CoA 8, CO2 and NADPH as catalysed by crotonyl-CoA carboxylase reductase (Ccr) (Fig. 2B).18,19 We therefore prepared Ccr,19 and used it to produce (2S)-5 or (2S)-4 from crotonyl-CoA(8) or acryloyl-CoA 9,18 respectively. When these products were then separately incubated with 2 and an appropriate CarB W79-based variant (i.e. CarB W79F or CarB W79A), we observed conversion of (2S)-5 or (2S)-4 to (6R)-7 or (6R)-6, respectively (Fig. 2B and 3).17 However, the CarB W79F/A variants convert (2R)-4/5 to (6S)-6/7 at a faster rate than converting (2S)-4/5 to (6R)-6/7 (Fig. 2B).17 We were therefore interested in selectively preparing the (2R)-alkylmalonyl-CoA epimers,17 and incubating them with CMPSs. We were also keen to extend the range of C6/C7 side chains tested with CMPS variants, which had been limited by the availability of malonyl coenzyme A derivatives.
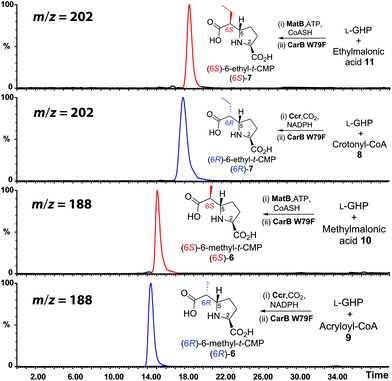 | ||
| Fig. 3 Stereoselective alkylation of L-GHP 2 employing enzyme pairs. The ion extracted LC-MS chromatograms (positive ionization mode) display the selectivities of Ccr/CarB W79F and MatB/CarB W79F for the (6R)-6/7 or (6S)-6/7 products, respectively. | ||
Incubation of C-2-alkylated malonic acid derivatives with L-GHPs, MatB and CMPS variants
(2R)-4 and (2R)-5 can be produced via reaction of 2-methylmalonic acid 10 and 2-ethylmalonic acid 11, respectively, with CoASH as catalysed by the malonyl-CoA synthetase MatB from Streptomyces coelicolor (Fig. 2C).20 The CarB Trp79 residue forms a part of the hydrophobic face of the CarB active site (Fig. 1B);17 we envisaged that substituting Trp79 for a less bulky residue e.g. Phe/Ala (Table 1) may enable acceptance of derivatives of 1 with bulky C-2 side chains, also available by MatB catalysis.|
|
|||||||
|---|---|---|---|---|---|---|---|
| Entry | Catalyst | Substrate | Product | d.r.a (R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S) S) |
Yieldb (%) | ||
| A (R) | B (n) | n | R | ||||
| a d.r.: diastereomeric ratio of epimers at C-6 or C-7 of 5- or 6-membered ring products, respectively. b Diastereomeric ratios and (isolated) yields were calculated as reported.15–17 c HFH: 4,4,5,5,6,6,6-heptafluorohexyl (HFH). 2,2-Dimethylmalonic acid (12) was also converted to the corresponding C-6 dimethyl-t-CMP derivative. | |||||||
| 1 | CarB wildtype | Methyl 10 | 1 | 1 | Methyl, (6S)-6 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
30 |
| 2 | CarB W79F | Methyl 10 | 1 | 1 | Methyl, (6S)-6 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
32 |
| 3 | CarB W79A | Methyl 10 | 1 | 1 | Methyl, (6S)-6 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
25 |
| 4 | CarB wildtype | Ethyl 11 | 1 | 1 | Ethyl, (6S)-7 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
3 |
| 5 | CarB W79F | Ethyl 11 | 1 | 1 | Ethyl, (6S)-7 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
17 |
| 6 | CarB W79A | Ethyl 11 | 1 | 1 | Ethyl, (6S)-7 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
19 |
| 7 | CarB W79F | Allyl 13 | 1 | 1 | Allyl, (6S)-28 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
14 |
| 8 | CarB W79A | Allyl 13 | 1 | 1 | Allyl, (6S)-28 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
13 |
| 9 | CarB W79F | Propyl 14 | 1 | 1 | Propyl, (6S)-29 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
19 |
| 10 | CarB W79A | Propyl 14 | 1 | 1 | Propyl, (6S)-29 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
30 |
| 11 | CarB W79A | Butyl 15 | 1 | 1 | Butyl, (6S)-30 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
9 |
| 12 | CarB W79A | Isobutyl 16 | 1 | 1 | Isobutyl, (6S)-31 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
9 |
| 13 | CarB W79A | Pentyl 17 | 1 | 1 | Pentyl, (6S)-32 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
27 |
| 14 | CarB W79A | 2-Methylbutyl 18 | 1 | 1 | 2-Methylbutyl, (6S)-33 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
6 |
| 15 | CarB W79A | Isopentyl 19 | 1 | 1 | Isopentyl, (6S)-34 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
32 |
| 16 | CarB W79A | Isoprenyl 20 | 1 | 1 | Isoprenyl, (6S)-35 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
36 |
| 17 | CarB W79A | Hexyl 21 | 1 | 1 | Hexyl, (6S)-36 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
30 |
| 18 | CarB W79A | HFHc22 | 1 | 1 | HFH, (6S)-37 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
5 |
| 19 | CarB W79A | Heptyl 23 | 1 | 1 | Heptyl, (6S)-38 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
10 |
| 20 | CarB W79A | Octyl 24 | 1 | 1 | Octyl, (6S)-39 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
9 |
| 21 | CarB wildtype | Methyl 10 | 2 | 2 | Methyl, (7S)-41 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
3 |
| 22 | CarB W79F | Methyl 10 | 2 | 2 | Methyl, (7S)-41 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
8 |
| 23 | CarB W79A | Methyl 10 | 2 | 2 | Methyl, (7S)-41 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
6 |
| 24 | CarB W79A | Ethyl 11 | 2 | 2 | Ethyl, (7S)-42 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
4 |
| 25 | CarB W79F | Ethyl 11 | 2 | 2 | Ethyl, (7S)-42 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
6 |
| 26 | CarB W79A | Allyl 13 | 2 | 2 | Allyl, (7S)-43 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
6 |
| 27 | CarB W79A | Propyl 14 | 2 | 2 | Propyl, (7S)-44 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
5 |
| 28 | CarB W79A | Butyl 15 | 2 | 2 | Butyl, (7S)-45 | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
3 |
We initially tested the MatB/CMPS pair with 10 and 11 (Table 1) as well as 2,2-dimethylmalonic acid 12 (results not shown). In each case, the MatB/wildtype CarB coupled reaction resulted in the production of the anticipated t-CMP derivative, as revealed by LC-MS analyses, in yields comparable to those resulting from direct incubation of the corresponding synthetic derivatives of 1 and 2 with wildtype CarB.17 The observed C-6 epimeric ratio in the case of both 10 and 11 reactions was ≥99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in favour of (6S)-6/7 (Table 1: entries 1 and 4). The MatB/CarB W79F/A coupled reactions were also selective for production of the (6S)-epimer of 6/7 and the isolated yields were either similar (Table 1: entries 2 and 3) or higher (Table 1: entries 5 and 6) than those for wildtype CarB.
1 in favour of (6S)-6/7 (Table 1: entries 1 and 4). The MatB/CarB W79F/A coupled reactions were also selective for production of the (6S)-epimer of 6/7 and the isolated yields were either similar (Table 1: entries 2 and 3) or higher (Table 1: entries 5 and 6) than those for wildtype CarB.
We then tested a series of C-2 mono-alkylated malonic acid derivatives ranging from those with a 3 carbon side-chain to derivatives with a 10 carbon side-chain, with varying saturation and/or substitution (Fig. S1,†Table 1). Analytical LC-MS assays revealed that the MatB/CarB W79A pair converts malonic acids with the following C-2 side chains: allyl (13), n-propyl (14), n-butyl (15), isobutyl (16), n-pentyl (17), 2-methylbutyl (18), isopentyl (19), isoprenyl (20), n-hexyl (21), 4,4,5,5,6,6,6-heptafluorohexyl (22), n-heptyl (23), and n-octyl (24) to form the corresponding 6-alkyl-5-carboxymethylproline derivatives (28–39) (Fig. S2,†Table 1). The C-2 malonic acid derivative with an n-decyl side chain (25) was a poor substrate for the coupled MatB/CarB W79A-catalysed reaction (the yield of the t-CMP (26) derivative was ∼6% of that for 6-n-octyl-t-CMP (39), Table 1, entry 20).
Scale-up and NMR of the LC-MS purified MatB/CarB W79A-catalysed products revealed that a single detectable epimer of 6-alkyl-t-CMP with the (5S,6S)-stereochemistry was formed with isolated yields from 5% to 36% (Table 1: entries 8, 10–20). (There is likely scope for optimization of the small-scale reactions, including in cells) LC-MS analyses of the reaction catalysed by MatB/wildtype CarB pair did not result in observation of detectable quantities of the alkylated products that were observed with the MatB/CarB W79A reactions. In the case of the MatB/CarB W79F reactions, we only observed formation of derivatives with allyl and n-propyl side-chains, at comparable yields to those of the MatB/CarB W79A-catalysed reaction (Table 1: entries 7, 9). The reduced promiscuity and/or efficiency for wildtype CarB and CarB W79F compared to CarB W79A is consistent with the predicted reduced steric demand of the latter (Fig. 1B, Table 1).
Incubation of C-2-alkylated malonic acid derivatives and L-aminoadipate semialdehyde, MatB and CMPS variants
We then investigated the ability of the MatB/CMPS pair for formation of C-7 alkylated carboxymethylpipecolic acid (CMPi) derivatives employing L-aminoadipate semialdehyde (L-AASA) (40). In the case of the MatB/wildtype CarB reactions, we observed (7S)-7-methyl-t-CMPi (41) formation, but in a lower yield than those for the CarB W79 variants (Table 1, entries 21–23). Use of MatB/CarB W79A enabled isolation of (7S)-7-methyl- (41), (7S)-7-ethyl- (42), (7S)-7-allyl- (43), (7S)-7-propyl- (44) and (7S)-7-butyl-(45)-t-CMPi derivatives (Fig. S4† and Table 1: entries 23, 24, 26–28). For 2D NMR of the MatB/CMPS-catalysed products, see Fig. S10 to S26.†Testing of malonyl CoA analogues
We also tested truncated and substituted malonyl CoA analogues. MatB catalyses formation of truncated malonyl-CoA analogues, i.e. malonyl-thioesters of pantetheine 46 and N-acetyl-cysteamine 47, from appropriate precursors20 (Fig. S5 and S6†).The MatB/CarB pair catalysed production of 3 from 2, using pantetheine (46) or N-acetyl cysteamine (47), substituting for CoASH, occurs in ∼20% or ∼15%, respectively, of the yields obtained with CoASH (Fig. S6†). When we tested C-2 alkylated derivatives with 46, in addition to the t-CMP derivative, we observed two previously undetected (by LC-MS, Fig. S5 and S7†) species, corresponding to the methyl ester- and pantetheinyl-t-CMP derivatives. The methyl ester is presumably produced in the methanol quenching step. In the case of the 2-isoprenyl malonic acid (20)/MatB/CarB-W79A system (Fig. 4), the assignment of the methyl ester (48) was confirmed by NMR (during isolation, the assigned pantetheinyl thioester (49) underwent hydrolysis to give (35)). Thus, in principle it is possible to use MatB/CMPSs for the regioselective production of t-CMP esters. These observations are consistent with the proposed intermediacy of an enzyme-bound t-CMP-CoA thioester (50) in CarB catalysis.4,5 It is possible that the observation (by LC-MS) of the thioester (49)/methyl ester (48) in the case of the C-2 substituted alkyl malonic acid derivatives, reflects steric hindrance in the hydrolysis step, resulting in release of the non-optimal pantetheinyl-thioester (49).
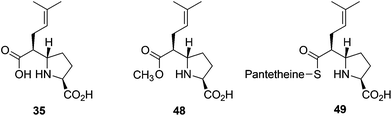 | ||
| Fig. 4 Products from the incubation of C-2 isoprenylmalonic acid (20) with MatB/CarB W79A using pantetheine as a replacement for coenzyme A. | ||
Chemoenzymatic preparation of malonyl-(dethia)CoA analogues and their testing as potential substrates for CMPSs
We also prepared and tested carba- (51) (CH2)-, amide- (52) and ester-(53) analogues of the thioester bond of malonyl coenzyme A (1)21–24 (Scheme S1†). Incubation of the amide analogue (52) did not lead to a detectable product. LC-MS studies reveal that the carba-analogue (51) is converted to the corresponding carba(dethia)-t-CMP-CoA derivative (54) (Fig. 5). The ester analogue (53) was converted to the corresponding oxa(dethia)CoA ester (55) and t-CMP (3). The detection of the former may reflect relatively slow hydrolysis of the ester compared to thioester intermediate. Overall, these results support the proposed CarB mechanism and further illustrate the capacity of CMPSs to accept substrate analogues.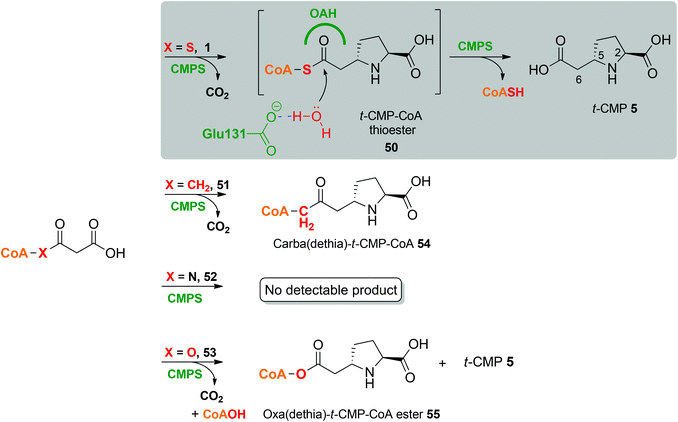 | ||
| Fig. 5 Malonyl-CoA analogues as CMPS substrates. | ||
Conclusions
There is a need to efficiently produce functionalised chiral heterocycles for use in medicinal chemistry and as catalysts. Whilst considerable efforts have been directed towards organic synthesis in this regard, less effort has been directed towards enzyme catalysis, particularly in a coupled sense. However, the use of cell-produced natural products for structure–activity relationship studies can be challenging, as it can be difficult to produce a desirable set of analogues, due to the limitations of working with multi-step processes in cells. An alternative approach is to couple reactions in vitro using purified (engineered) enzymes and substrate analogues, bearing in mind that selected coupled reaction sequences could be introduced into cells at a later stage, when larger scale production is required. Our results in which the stereocontrolled production of C-2 malonyl-CoA derivatives, from “achiral” precursors, is coupled to heterocycle formation using CMPS variants demonstrate how a set of chiral 5- and 6-membered ring heterocycles can be efficiently prepared in two enzyme-catalysed steps (Fig. S9†). It is notable that near complete control of stereochemistry at C-6/C-7 of t-CMP 5/t-CMPi can be achieved by appropriate choice of the alkylmalonyl-CoA synthesising enzyme (Ccr or MatB, Fig. S9†) and reaction conditions. Additional diversity might be introduced by the use of (truncated) malonyl-CoA analogues (substituted at C-2) or by using different alcohols to quench the reaction. Thus, we believe that one productive future direction could be the de novo construction of unnatural biosynthetic pathways, for the production of both natural product-like and -unlike structures.Experimental details and spectroscopic characterisations are given in the ESI.†
We thank the Biotechnology and Biological Sciences Research Council, Berrow Foundation (LH) and CONACyT and FIDERH (Mexico, RGC) for funding. The MatB expression plasmid was kindly provided by Dr A. T. Keatinge-Clay. The expression plasmids of the CoA biosynthesis enzymes were kindly provided by Dr M. Tosin. We thank Sven Warhaut for his help during the course of the work.
Notes and references
- S. K. Panday, Tetrahedron: Asymmetry, 2011, 22, 1817–1847 CrossRef CAS PubMed
.
- M. C. Sleeman, J. L. Sorensen, E. T. Batchelar, M. A. McDonough and C. J. Schofield, J. Biol. Chem., 2005, 280, 34956–34965 CrossRef CAS PubMed
.
- M. C. Sleeman and C. J. Schofield, J. Biol. Chem., 2004, 279, 6730–6736 CrossRef CAS PubMed
.
- B. Gerratana, S. O. Arnett, A. Stapon and C. A. Townsend, Biochemistry, 2004, 43, 15936–15945 CrossRef CAS PubMed
.
- E. T. Batchelar, R. B. Hamed, C. Ducho, T. D. W. Claridge, M. J. Edelmann, B. Kessler and C. J. Schofield, Angew. Chem., Int. Ed., 2008, 47, 9322–9325 CrossRef CAS PubMed
.
- L. E. Nunez, C. Mendez, A. F. Brana, G. Blanco and J. A. Salas, Chem. Biol., 2003, 10, 301–311 CrossRef CAS
.
- R. B. Hamed, E. T. Batchelar, J. Mecinovic, T. D. W. Claridge and C. J. Schofield, ChemBioChem, 2009, 10, 246–250 CrossRef CAS PubMed
.
- M. J. Bodner, R. Li, R. M. Phelan, M. F. Freeman, K. A. Moshos, E. P. Lloyd and C. A. Townsend, ChemBioChem, 2011, 12, 2159–2165 CrossRef CAS PubMed
.
- R. B. Hamed, E. T. Batchelar, I. J. Clifton and C. J. Schofield, Cell. Mol. Life Sci., 2008, 65, 2507–2527 CrossRef CAS PubMed
.
- R. B. Hamed, J. R. Gomez-Castellanos, L. Henry, C. Ducho, M. A. McDonough and C. J. Schofield, Nat. Prod. Rep., 2013, 30, 21–107 RSC
.
- P. Schultz and R. Lerner, Science, 1995, 269, 1835–1842 CAS
.
- J. Reymond, J. Mol. Catal. B: Enzym., 1998, 5, 331–337 CrossRef CAS
.
- S. Dean, W. Greenberg and C. Wong, Adv. Synth. Catal., 2007, 349, 1308–1320 CrossRef CAS
.
- A. Bolt, A. Berry and A. Nelson, Arch. Biochem. Biophys., 2008, 474, 318–330 CrossRef CAS PubMed
.
- R. B. Hamed, J. Mecinovic, C. Ducho, T. D. W. Claridge and C. J. Schofield, Chem. Commun., 2010, 46, 1413–1415 RSC
.
- R. B. Hamed, L. Henry, J. R. Gomez-Castellanos, J. Mecinović, C. Ducho, J. L. Sorensen, T. D. W. Claridge and C. J. Schofield, J. Am. Chem. Soc., 2012, 134, 471–479 CrossRef CAS PubMed
.
- R. B. Hamed, J. R. Gomez-Castellanos, A. Thalhammer, D. Harding, C. Ducho, T. D. W. Claridge and C. J. Schofield, Nat. Chem., 2011, 3, 365–371 CrossRef CAS PubMed
.
- T. J. Erb, V. Brecht, G. Fuchs, M. Muller and B. E. Alber, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 8871–8876 CrossRef CAS PubMed
.
- T. J. Erb, I. A. Berg, V. Brecht, M. Muller, G. Fuchs and B. E. Alber, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 10631–10636 CrossRef CAS PubMed
.
- A. J. Hughes and A. Keatinge-Clay, Chem. Biol., 2011, 18, 165–176 CrossRef CAS PubMed
.
- M. Tosin, D. Spiteller and J. B. Spencer, ChemBioChem, 2009, 10, 1714–1723 CrossRef CAS PubMed
.
- M. Tosin, Y. Demydchuk, J. S. Parascandolo, C. B. Per, F. J. Leeper and P. F. Leadlay, Chem. Commun., 2011, 47, 3460–3462 RSC
.
- I. Nazi, K. P. Koteva and G. D. Wright, Anal. Biochem., 2004, 324, 100–105 CrossRef CAS PubMed
.
- M. Tosin, L. Betancor, E. Stephens, W. M. A. Li, J. B. Spencer and P. F. Leadlay, ChemBioChem, 2010, 11, 539–546 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ob41525b |
| This journal is © The Royal Society of Chemistry 2013 |