 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structure–activity relationships in Toll-like receptor 7 agonistic 1H-imidazo[4,5-c]pyridines†
Euna
Yoo
,
Breanna M.
Crall
,
Rajalakshmi
Balakrishna
,
Subbalakshmi S.
Malladi
,
Lauren M.
Fox
,
Alec R.
Hermanson
and
Sunil A.
David
*
Department of Medicinal Chemistry, University of Kansas, Multidisciplinary Research Building, Room 320D, 2030 Becker Drive, Lawrence, KS 66047, USA. E-mail: sdavid@ku.edu; Fax: +1 785-864-1961; Tel: +1 785-864-1610
First published on 15th August 2013
Abstract
Engagement of TLR7 in plasmacytoid dendritic cells leads to the induction of IFN-α/β which plays essential functions in the control of adaptive immunity. We had previously examined structure–activity relationships (SAR) in TLR7/8-agonistic imidazoquinolines with a focus on substituents at the N1, C2, N3 and N4 positions, and we now report SAR on 1H-imidazo[4,5-c]pyridines. 1-Benzyl-2-butyl-1H-imidazo[4,5-c]pyridin-4-amine was found to be a pure TLR7-agonist with negligible activity on TLR8. Increase in potency was observed in N6-substituted analogues, especially in those compounds with electron-rich substituents. Direct aryl–aryl connections at C6 abrogated activity, but TLR7 agonism was reinstated in 6-benzyl and 6-phenethyl analogues. Consistent with the pure TLR7-agonistic behavior, prominent IFN-α induction in human PBMCs was observed with minimal proinflammatory cytokine induction. A benzologue of imidazoquinoline was also synthesized which showed substantial improvements in potency over the parent imidazopyridine. Distinct differences in N6-substituted analogues were observed with respect to IFN-α induction in human PBMCs on the one hand, and CD69 upregulation in lymphocytic subsets, on the other.
Introduction
Host responses to pathogens are mediated via highly coordinated mechanisms involving both the innate and adaptive limbs of the immune system. The innate immune system utilizes germline-encoded pattern recognition receptors (PRRs) to detect pathogen-associated molecular patterns (PAMPs) that are distinct and unique to the pathogen.1–3 PRRs encompass a broad range of molecules4 that are secreted into the extracellular environment (such as the collectins,5 ficolins,6 pentraxins,7 alarmins8), exist in the cytosol (examples of which include the retinoic acid-inducible gene I-like receptors,9 and the nucleotide-binding domain and leucine-rich repeat-containing receptors10), or are present on membranes.Important among the transmembrane PRRs include the Toll-like receptors11 (TLRs) which are either expressed on the plasma membrane or in the endolysosomal compartments.1,12 At least 10 functional TLRs are encoded in the human genome, each with an extracellular domain having leucine-rich repeats (LRR) and a cytosolic domain called the Toll/IL-1 receptor (TIR) domain.13 The ligands for these receptors are highly conserved microbial molecules such as lipopolysaccharides (LPS) (recognized by TLR4), lipopeptides (TLR2 in combination with TLR1 or TLR6), flagellin (TLR5), single stranded RNA (TLR7 and TLR8), double stranded RNA (TLR3), CpG motif-containing DNA (recognized by TLR9), and profilin present on uropathogenic bacteria (TLR11).14,15 TLR1, -2, -4, -5, and -6 recognize extracellular stimuli, while TLR3, -7, -8 and -9 function within the endolysosomal compartment.13 The activation of TLRs by their cognate ligands leads to production of inflammatory cytokines, and up-regulation of major histocompatibility complex (MHC) molecules and co-stimulatory signals in antigen-presenting cells as well as activating natural killer (NK) cells (innate immune response), which leads to the priming and amplification of T-, and B-cell effector functions (adaptive immune responses).16–19
The Type I interferon (IFN) family in humans include approximately 20 IFN-α subtype genes in addition to individual genes encoding IFN-β, -κ, -ε and -ω; these monomeric secreted proteins bind to a single IFN-α/β receptor, which is constitutively expressed in virtually all cell types.20 Occupancy of TLR721–23 or TLR924,25 in professional antigen-presenting cells (APCs), particularly plasmacytoid dendritic cells (pDCs), leads to the induction of IFN-α/β. Although the Type I IFNs are best known historically for their antiviral activities,26 recent studies show that they have many essential functions in the control of adaptive immunity.2 First, Type I IFNs promote cross-priming through direct stimulation of DCs, leading to specific CD8+ lymphocytic responses to soluble antigens.27 Second, Type I IFNs potently enhance the primary antibody responses to soluble antigens, inducing sustained and durable humoral responses with appropriate isotype switching, as well as the induction of immunological memory.28 B lymphocytes can differentiate into two distinct types of functionally polarized effectors: B-effector-1-cells (Be-1), producing a Th-1-like cytokine pattern, or Be-2, characterized by a Th-2-like profile.29,30 It is of particular interest that recent reports suggest that IFN-α may serve as an initial trigger for Be-1-biased differentiation pattern.31 Third, Type I interferons secondarily induce Type II IFN (IFN-γ) secretion, also driving Th-1-biased adaptive immune responses.32 Type I IFN-inducing TLR ligands may therefore hold promise as vaccine adjuvants.
In an effort to identify optimal immunostimulatory chemotypes, we have screened representative members of virtually the entire compendium of known TLR agonists in a series of hierarchical assays including primary TLR-reporter assays, secondary indices of immune activation such as IFN-α/β/γ and cytokine induction, activation of lymphocytic subsets in whole human blood, and tertiary screens characterizing transcriptomal activation patterns.33 In these assays, small-molecule agonists of TLR7 were uniquely immunostimulatory; they were potent inducers of Type I IFN and, unlike TLR-4, -5, or -8 agonists,33 did not evoke dominant proinflammatory cytokine responses, suggesting that they may be effective, yet safe vaccine adjuvants, a premise that we have been actively exploring.34 Small molecule TLR7 agonists are also being investigated as orally bioavailable, endogenous Type I IFN inducers for the management of chronic viral diseases,35 especially hepatitis C and hepatitis B. Current therapeutic regimens for the therapy of hepatitis C and hepatitis B include parenteral IFN-α.36 Clinical trials of TLR7 agonists for hematological malignancies are also currently underway.37
The currently known small molecule agonists of TLR7 occupy a very small chemical space, and are represented by the 1H-imidazo[4,5-c]quinolines,38 8-hydroxy-39–41 or 8-oxoadenines,42 and guanine nucleoside analogues.43,44 We had previously reported structure–activity relationships (SAR) in the imidazoquinolines with a focus on substituents at the N1, C2, N3 and N4 positions,45,46 and we had observed that relatively minor structural modifications at these positions yielded compounds with widely differing immunomodulatory properties.34,47–49 It was of interest, therefore, to extend our SAR studies to the quinoline ring system. We asked if a part-structure (imidazopyridine) or a benzologue (benzoimidazoquinoline) would alter the biological properties of the parent imidazoquinoline compound. Examination of the structures of 3M-00350 and the 8-hydroxy- and 8-oxoadenines (Fig. 1) suggested that the quinoline system may be dispensable, and activity would be retained in imidazopyridines. Indeed, imidazopyridine derivatives with alkyl groups at C6 and C7 positions,51 hydroxyalkyl,52 oxime and hydroxylamine-bearing substituents53 at C2, and alkylsulfonamide substituents at the N1 position54 have been reported in the patent literature. Detailed activity profiles of these compounds, however, are not available, perhaps owing to the fact that the investigations of such compounds precede the discovery of the TLRs.
![Structures of small molecule agonists of TLR7 represented by the 8-oxoadenine (SM360320, ref. 42), 8-hydroxyadenines (CL264, ref. 39), 1H-imidazo[4,5-c]quinolines 3M-003 (ref. 49), 1-isobutyl-1H-imidazo[4,5-c]quinolin-4-amine (Imiquimod) and 1-benzyl-2-butyl-1H-imidazo[4,5-c]quinolin-4-amine (IMDQ, ref. 45).](/image/article/2013/OB/c3ob40816g/c3ob40816g-f1.gif) | ||
| Fig. 1 Structures of small molecule agonists of TLR7 represented by the 8-oxoadenine (SM360320, ref. 42), 8-hydroxyadenines (CL264, ref. 39), 1H-imidazo[4,5-c]quinolines 3M-003 (ref. 49), 1-isobutyl-1H-imidazo[4,5-c]quinolin-4-amine (Imiquimod) and 1-benzyl-2-butyl-1H-imidazo[4,5-c]quinolin-4-amine (IMDQ, ref. 45). | ||
Incorporating substituents that we had previously determined to be optimal in the imidazoquinolines (N1-benzyl and C2-butyl; IMDQ, Fig. 1), we embarked on the syntheses and biological evaluation of novel 1H-imidazo[4,5-c]pyridine analogues with modifications at the N4- and C6 positions. The parent imidazopyridine compound, 1-benzyl-2-butyl-1H-imidazo[4,5-c]pyridin-4-amine, exhibited moderate TLR7-agonistic activity. N4-Acyl or -alkyl substitutions abrogated activity. The majority of C6 derivatives bearing aryl groups were also inactive, but analogues with N6-benzyl substituents gained TLR7-specific activity. Particular N6 substituents were found to augment TLR7-specific agonistic potency without compromising specificity at TLR7; consistent with their pure TLR7 activity (and undetectable TLR8 agonism), these compounds potently induced IFN-α in human peripheral blood mononuclear cells (PBMCs), upregulated CD69 in lymphocytic subsets, and yet showed very weak proinflammatory cytokine-inducing activities. Strong Type I IFN inducers, especially in conjunction with attenuated proinflammatory profiles are expected to be potently adjuvantic without inducing prominent local or systemic inflammation.
Results and discussion
Our interest in exploring TLR7 agonists as vaccine adjuvants has been greatly reinforced by our observations that pure TLR7 agonists, unlike other TLR ligands, are potently immunostimulatory without prominently activating inflammatory programs in human whole blood model systems.55 As mentioned earlier, the structures of 3M-00350 and the 8-hydroxy- and 8-oxoadenines (Fig. 1), as well as patent literature suggested that the quinoline system may be dispensable, and activity would be retained in imidazopyridines. Our previous SAR studies on the imidazoquinolines had established that N1-benzyl and C2-butyl substituents were optimal;46 our point of departure in examining structure–activity relationships in the imidazopyridines consequently began with the evaluation of 1-benzyl-2-butyl-1H-imidazo[4,5-c]pyridin-4-amine (5), following the synthetic strategy described earlier (Scheme 1).38,46 Compound 5, itself a novel and unprecedented structure, was found to possess TLR7-specific agonistic activity (EC50: 1.57 μM, Fig. 2, Table 1), with negligible TLR8 activity. The potency of the lead TLR7-specific imidazoquinoline (1-benzyl-2-butyl-1H-imidazo[4,5-c]quinolin-4-amine, structure in Fig. 1) was 0.06 μM (Fig. 2).46 | ||
| Scheme 1 Reagents: (i) BnNH2, NEt3, CH2Cl2; (ii) Zn, HCOONH4, MeOH; (iii) a. C4H9COCl, NEt3, THF; b. NaOH, EtOH; (iv) mCPBA, CHCl3; (v) a. Benzoyl isocyanate, CH2Cl2; b. NaOMe, MeOH. | ||
 | ||
| Fig. 2 TLR7 agonistic activity of imidazopyridine compounds. Data points represent means and standard deviations on quadruplicates. | ||
|
|
|||
|---|---|---|---|
| Compound number | R1 | R2 | hTLR7-agonistic activity (μM) |
| a Inactive: no activity was detected up to a concentration of 500 μg mL−1. | |||
| 5 | H | H | 1.57 |
| 6a | COCH3 | H | Inactivea |
| 6b | COC3H7 | H | Inactive |
| 11a | C4H9 | H | Inactive |
| 11b | CH2C6H5 | H | Inactive |
| 17 | H | Cl | Inactive |
| 19a | H | NH2 | 1.25 |
| 19b | H | NHC4H9 | 0.34 |
| 19c | H | NHC7H15 | 0.76 |
| 19d | H |

|
0.32 |
| 19e | H | NHC6H5 | 1.72 |
| 19f | H | NHCH2C6H5 | 0.21 |
| 19g | H |

|
0.075 |
| 19h | H |

|
0.61 |
| 19i | H |
|
1.04 |
| 19j | H |
|
0.64 |
| 19k | H |

|
0.075 |
| 19l | H |

|
0.25 |
| 19m | H |

|
0.25 |
| 19n | H |
|
Inactive |
| 19o | H |

|
1.09 |
| 19p | H |

|
0.26 |
| 19q | H |

|
0.37 |
| 19r | H |

|
1.08 |
| 19s | H |
|
Inactive |
| 23a | H | C4H9 | 0.28 |
| 23b | H | C6H5 | Inactive |
| 23c | H |

|
Inactive |
| 23d | H |

|
Inactive |
| 23e | H |
|
Inactive |
| 23f | H |

|
Inactive |
| 23g | H |

|
0.28 |
| 23h | H |
|
1.80 |
| 23i | H |
|
Inactive |
| 23j | H |

|
0.57 |
| 30 | Benzologue (Scheme 7) | 0.22 | |
Acylation (6a, 6b, Scheme 2) of the C4-NH2 resulted in complete abrogation of activity (Table 1). C6-modified analogues were synthesized via an alternate route. Nitration of 4-amino-2-chloropyridine resulted, as expected, in a mixture of the 3- and 5-nitro intermediates 7a and 7b, which were taken forward to obtain the 4- and 6-chloroimidazopyridines 10a and 10b (Scheme 3). Excellent chromatographic separation of these advanced intermediates was possible. Pd-catalyzed C–N cross-coupling reactions using n-butylamine and benzylamine furnished the C4-N-alkylated analogues 11a and 11b, respectively (Scheme 3). A 4-butoxy analogue 11c was also obtained by ipso-chloro displacement with 1-butanol. Compounds 11a–c were, however, inactive (Table 1). We had envisaged utilizing the 6-chloro imidazopyridine intermediate 10b for synthesizing C6-functionalized analogues. However, this intermediate exhibited unexpectedly low reactivity to displacement with nucleophiles or to Buchwald–Hartwig coupling reactions. As outlined in Scheme 4, we utilized 4-amino-2,6-dichloropyridine as the starting material and obtained 13 as a key intermediate which, upon reaction with tert-octylamine56 provided exclusively the N2-alkylated intermediate 14. The 6-chloro-N-(2,4,4- trimethylpentan-2-yl)-1H-imidazopyridin-4-amine intermediate 16 was obtained without difficulty, and we were able to synthesize the N6-substituted analogues 19a–o under conventional Buchwald–Hartwig conditions (Scheme 4). TLRs signal via ligand-induced dimerization, but since that the crystal structure of human TLR7 and of its ligand binding modes are as yet unknown, we utilized intermediate 16 in constructing ‘dimeric’ imidazopyridines (using p- and m-xylylenediamine, Scheme 5) to ascertain if such pre-organized dimeric ligands could yield high-potency agonists. For the C6-substituted compounds 23a–j (Scheme 6), we observed mediocre yields in pilot Suzuki coupling reactions with the advanced intermediate 16. We therefore exploited the electron-withdrawing resonance effect of the 3-nitro group in 14. As expected, the classical Suzuki reaction on intermediate 14 using various aliphatic and aromatic boronic acids/boronate esters resulted in the intermediates 20a–j (Scheme 6), which were further derivatized to obtain the desired C6 alkyl/aryl substituted imidazopyridines 23a–j.
 | ||
| Scheme 2 Reagents: (i) RCOCl, NEt3, CH2Cl2. | ||
 | ||
| Scheme 3 Reagents: (i) a. H2SO4, HNO3; b. H2SO4; (ii) BnBr, NaH, THF; (iii) Zn, HCOONH4, MeOH; (iv) a. C4H9COCl, NEt3, THF; b. NaOH, EtOH; (v) For 11a and 11b, amines (n-BuNH2 and BnNH2, respectively), Pd2(dba)3, DavePhos, KOtBu, dioxane; For 11c, BuOH, NaH, THF. | ||
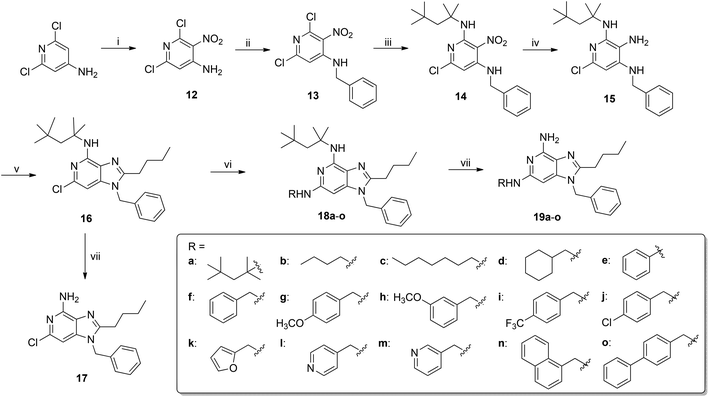 | ||
| Scheme 4 Reagents: (i) a. H2SO4, HNO3; b. H2SO4; (ii) BnBr, NaH, THF; (iii) t-Octylamine, NEt3, CH2Cl2; (iv) Zn, HCOONH4, MeOH; (v) a. C4H9COCl, NEt3, THF; b. NaOH, EtOH; (vi) RNH2, Pd2(dba)3, DavePhos, KOtBu, dioxane; (vii) HCl. | ||
 | ||
| Scheme 5 Reagents: (i) Pd2(dba)3, DavePhos, KOtBu, dioxane; (ii) HCl. | ||
 | ||
| Scheme 6 Reagents: (i) For 20a–f and 20j, R-boronic acid, Pd(dppf)Cl2, Cs2CO3, dioxane; for 20c, 4-cyanophenyl boronic acid, Pd(dppf)Cl2, Cs2CO3, dioxane; for 20g–i, R-boronic acid pinacol ester, Pd(dppf)Cl2, Cs2CO3, dioxane; (ii) Zn, HCOONH4, MeOH; (iii) a. C4H9COCl, NEt3, THF; b. NaOH, EtOH; (iv) HCl. | ||
The 6-chloro imidazopyridine, 17 (Scheme 4) was inactive (Table 1). Buchwald–Hartwig-derived N6-substituted analogues 19a–q, however, showed a distinctive SAR. Compound 19a with a free NH2 at C6, obtained by coupling the tert-octylamine and subsequent N-dealkylation with HCl (Scheme 4, Table 1) displayed TLR7-specific agonism with a potency comparable to that of the parent C6-unsubstituted compound 5.
Modest gains in potency were obtained in analogues with short aliphatic substituents with N6-butyl (19b) and N6-cyclohexylmethyl (19d), but potency diminished in the N6-heptyl analogue (19c). The N6-phenyl-substituted compound 19e was marginally weaker than 5; however, the potency of the N6-benzyl analogue 19f was ∼7.6 times that of 5 (Table 1, Fig. 2), triggering a detailed SAR investigation on various aryl substituents at N6. Both steric and electronic effects appear to play a role in governing TLR7-agonistic potency, since the biphenylmethyl-substituted compound 19o was active, whereas the naphthylmethyl analogue 19n was quiescent; to a first approximation, electron-rich N6 substituents appear to be preferred, with the methoxybenzyl derivatives (19g and 19h) and the pyridinylmethyl compounds (19l and 19m) being marginally more active than the trifluoromethyl-(19i) or chloro-(19j) substituted analogues. Compounds 19p and 19q were also active in primary screens, with EC50 values of 0.26 and 0.37 μM, respectively (Table 1). In the C6-alkyl or -aryl analogues (Scheme 6), the SAR appeared more stringent. Whereas the C6-butyl compound 23a was more active than 5, direct aryl–aryl connections at C6 (23b–f) abrogated activity, but TLR7 agonistic properties were restored in the 6-benzyl (23g) and 6-phenethyl analogues (23j). Taken together with activity data of compounds of the 19 series, we surmised that rotational constraints about the C6-aryl groups may hinder TLR7 occupancy. Unlike TLR2, TLR3, TLR4,57 and TLR558 for which crystal structures are available as complexes with their cognate ligands, a detailed structural characterization of the mode of binding of TLR7 ligands is not yet available to guide structure-based design of small molecule agonists of TLR7, necessitating classical SAR approaches to refine successive iterations of ligand design.
The benzologue 30 was synthesized as shown in Scheme 7. It showed substantial improvements in potency over the parent imidazopyridine 5 (Fig. 3, Table 1), but the two most potent compounds in the entire series as adjudged by primary screens were the N6-(4-methoxybenzyl) and N6-(furan-2-ylmethyl) analogues (19g and 19k, respectively), both of which were approximately twenty-fold more potent than 5 (Fig. 2, Table 1).
 | ||
Scheme 7 Reagents: (i) a. HCl, HON![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2NO2, H2O; b. (CH3CO)2O, CH3COOK; (ii) POCl3; (iii) BnNH2, NEt3, CH2Cl2; (iv) Zn, HCOONH4, MeOH; (v) a. C4H9COCl, NEt3, THF; b. NaOH, EtOH; (vi) mCPBA, CH2Cl3, CHCl3, MeOH; (vii) a. Benzoyl isocyanate, CH2Cl2; b. NaOMe, MeOH. CHCH2NO2, H2O; b. (CH3CO)2O, CH3COOK; (ii) POCl3; (iii) BnNH2, NEt3, CH2Cl2; (iv) Zn, HCOONH4, MeOH; (v) a. C4H9COCl, NEt3, THF; b. NaOH, EtOH; (vi) mCPBA, CH2Cl3, CHCl3, MeOH; (vii) a. Benzoyl isocyanate, CH2Cl2; b. NaOMe, MeOH. | ||
 | ||
| Fig. 3 Dose–response profiles of TLR7 agonistic activity of compounds 5 and 30. Data points represent means and standard deviations on quadruplicates. | ||
We chose the nine most active compounds (19b, 19d, 19f–g, 19k–m, 19p and 23a) for evaluation in secondary screens using IFN-α and cytokine release in human PBMCs. We used, as reference compounds, imiquimod, a known TLR7 agonist,59,60 as well as CL07561,62 (2-propylthiazolo[4,5-c]quinolin-4-amine), a predominantly TLR8-active agonist with an EC50 of 1.32 μM in hTLR8 assays.63 Given that the imidazopyridine compounds are pure TLR7 agonists, we expected to find prominent IFN-α induction,33 and this was indeed the case, with 19p, 19m and 19k being the most potent (EC50: 0.3 μM, 0.4 μM and 0.7 μM, respectively; Fig. 4). CL075 was among the least potent in IFN-α induction (EC50: 2.6 μM; Fig. 4), and as expected for a TLR8 agonist, CL075 was dramatically more active in inducing proinflammatory cytokines such as TNF-α, IL-1β, and IL-8 (Fig. 4). We do not yet understand the basis for the slight discrepancy between rank-order potency in primary screens (19k ≈19 g >19f >19p; Fig. 2, Table 1) vis-à-vis IFN-α-inducing potency in human PBMCs (19p ≈19m >19k; Fig. 4), and we surmise that analogues with more basic C6 substituents may allow for higher endolysosomal partitioning. The dose–response profiles show characteristic biphasic responses (dose-dependent activation, followed by apparent suppression) as we had previously observed in several chemotypes. We verified that the apparent suppression was not due to cytotoxicity using LDH release and mitochondrial redox-based assays.
 | ||
| Fig. 4 Dose–response profiles of Type I interferon (IFN-α) and proinflammatory cytokine (IL-8, IL-1β, and TNF-α) induction by selected imidazopyridine (and reference) compounds. Representative data from three independent experiments are presented. | ||
We had previously shown that of all the various classes of innate immune stimuli, TLR7 agonists were extraordinarily immunostimulatory, stimulating virtually all subsets of lymphocytes (assessed by quantifying CD69 expression), and yet without inducing dominant proinflammatory cytokine responses,33 and we wished to confirm the rank-order potency observed in IFN-α induction assays described above. We observed considerable dissociation between Type I IFN induction on the one hand (Fig. 4), and CD69 upregulation in lymphocytic subsets on the other (Fig. 5). Whereas the subset of active compounds induced IFN-α with similar potencies (EC50 values between 0.3–2 μM; Fig. 4), pronounced differences were observed in CD69 expression in natural killer, cytokine-induced killer and B lymphocytic subsets with 19p being as active as the reference TLR7 agonist IMDQ, and 19d showing virtually no activity (Fig. 5). Possible mechanisms underlying the differential activity in these two compounds are being investigated.
 | ||
| Fig. 5 CD69 upregulation in human natural killer (NK), cytokine-induced killer (CIK) and nominal B lymphocytes by select imidazopyridine (and reference) compounds. | ||
Conclusions
These findings raise the possibility of utilizing these compounds in selectively targeting Type I IFN induction versus lymphocytic activation, and are being explored in greater detail.The potential advantages of strong Type I IFN inducers as candidate vaccine adjuvants have been discussed earlier. Such compounds, especially in conjunction with attenuated proinflammatory cytokines, are expected to be potently adjuvantic without inducing prominent local or systemic inflammation. As mentioned earlier, the prominent Type I IFN inducing abilities of the imidazopyridines may also find utility as an alternative therapeutic strategy to address disease states wherein systemic IFN-α is of proven benefit. A clear delineation of structural features that confer TLR specificity not only charts a rational course for the development of effective, yet safe vaccine adjuvants, but also provides tools to understand innate immune function in greater detail.
Experimental
Materials and equipment
All of the solvents and reagents used were obtained commercially and used as such unless noted otherwise. Moisture- or air-sensitive reactions were conducted under nitrogen atmosphere in oven-dried (120 °C) glass apparatus. The solvents were removed under reduced pressure using standard rotary evaporators. Flash column chromatography was carried out using RediSep Rf ‘Gold’ high performance silica columns on CombiFlash Rf instrument unless otherwise mentioned, while thin-layer chromatography was carried out on silica gel (200 µm) CCM pre-coated aluminum sheets. Purity for all final compounds was confirmed to be greater than 97% by LC-MS using a Zorbax Eclipse Plus 4.6 mm × 150 mm, 5 µm analytical reverse phase C18 column with H2O-isopropanol or H2O–CH3CN gradients and an Agilent 6520 ESI-QTOF Accurate Mass spectrometer (mass accuracy of 5 ppm) operating in the positive ion (or negative ion, as appropriate) acquisition mode.Synthesis of compound 1: N-benzyl-3-nitropyridin-4-amine
To a solution of 4-chloro-3-nitropyridine (1.0 g, 6.31 mmol) in 25 mL of CH2Cl2 were added triethylamine (1.32 mL, 9.47 mmol) and benzyl amine (0.83 mL, 7.57 mmol). The reaction mixture was refluxed for 18 h. The solvent was then evaporated under vacuum and H2O was added to the residue. The solution was extracted with CH2Cl2 (3 × 20 mL), washed with water and dried over sodium sulfate. The solvent was evaporated and the residue was purified using silica gel column chromatography (5% MeOH–CH2Cl2) to obtain compound 1 as a yellow solid (1.4 g, 94%). 1H NMR (500 MHz, CDCl3) δ 9.22 (s, 1H), 8.53 (s, 1H), 8.25 (dd, J = 6.1, 0.6 Hz, 1H), 7.41–7.35 (m, 2H), 7.32 (dd, J = 7.2, 5.3 Hz, 3H), 6.69 (d, J = 6.2 Hz, 1H), 4.56 (d, J = 5.7 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 153.38, 149.06, 148.68, 135.96, 130.04, 129.21, 128.22, 127.10, 108.25, 46.85. MS (ESI) calculated for C12H12N3O2, m/z 230.0924, found 230.0949 (M + H)+.Synthesis of compound 2: N4-benzylpyridine-3,4-diamine
To a solution of compound 1 (1.0 g, 4.36 mmol) in 40 mL of MeOH were added zinc dust (1.4 g, 21.8 mmol) and ammonium formate (1.4 g, 21.8 mmol). The reaction mixture was stirred at room temperature for 10 min and filtered through celite. Then the solvent was evaporated and the residue was dissolved in water. This was extracted with EtOAc (3 × 20 mL), washed with water and dried over sodium sulfate. The solvent was evaporated under vacuum to obtain the compound 2 (0.8 g, 92%). 1H NMR (500 MHz, CDCl3) δ 7.81 (d, J = 5.4 Hz, 1H), 7.77 (s, 1H), 7.29–7.19 (m, 5H), 6.36 (d, J = 5.4 Hz, 1H), 4.87 (s, 1H), 4.28 (d, J = 5.4 Hz, 2H), 3.28 (s, 2H). 13C NMR (126 MHz, CDCl3) δ 144.52, 143.51, 138.22, 137.53, 128.96, 128.79, 127.55, 127.48, 105.29, 47.22. MS (ESI) calculated for C12H14N3, m/z 200.1182, found 200.1242 (M + H)+.Synthesis of compound 3: 1-benzyl-2-butyl-1H-imidazo[4,5-c]pyridine
To a solution of compound 2 (400 mg, 2.00 mmol) in 20 mL of anhydrous THF were added triethylamine (0.29 mL, 2.10 mmol) and valeryl chloride (0.27 mL, 2.20 mmol). The reaction mixture was refluxed for 2 h. The solvent was then removed under vacuum, and the residue was dissolved in EtOAc and washed with water. The EtOAc fraction was dried using sodium sulfate and evaporated under vacuum to obtain the intermediate amide compound. This was dissolved in 20 mL of EtOH and NaOH (160 mg, 4.00 mmol) in 2 mL of H2O was added. The reaction mixture was refluxed for 4 h. The solvent was then removed under vacuum, and the residue was dissolved in EtOAc and washed with water. The organic layer was dried using sodium sulfate and evaporated and purified using column chromatography (5% MeOH–CH2Cl2) to obtain the compound 3 (210 mg, 40%). 1H NMR (500 MHz, CDCl3) δ 9.04 (d, J = 0.6 Hz, 1H), 8.34 (d, J = 5.6 Hz, 1H), 7.34–7.28 (m, 3H), 7.14 (dd, J = 5.6, 1.0 Hz, 1H), 7.01 (dd, J = 7.7, 1.8 Hz, 2H), 5.33 (s, 2H), 2.86–2.81 (m, 2H), 1.82 (dt, J = 15.5, 7.7 Hz, 2H), 1.46–1.36 (m, 2H), 0.91 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 157.30, 142.14, 142.00, 140.48, 140.01, 135.26, 129.28, 128.37, 126.27, 105.12, 47.25, 29.44, 27.38, 22.61, 13.85. MS (ESI) calculated for C17H20N3, m/z 266.1652, found 266.1715 (M + H)+.Synthesis of compound 4: 1-benzyl-2-butyl-1H-imidazo[4,5-c]pyridine 5-oxide
To a solution of compound 3 (210 mg, 0.79 mmol) in 15 mL of was added m-chloroperoxybenzoic acid (443 mg, 1.98 mmol), and the solution was refluxed at 45–50 °C for 1 h. The solvent was then removed and the residue was purified using column chromatography (10% MeOH–CH2Cl2) to obtain the N-oxide derivative (188 mg, 85%). 1H NMR (500 MHz, CDCl3) δ 8.71 (d, J = 1.3 Hz, 1H), 8.05 (dd, J = 7.0, 1.6 Hz, 1H), 7.36–7.30 (m, 3H), 7.01 (dd, J = 10.4, 4.3 Hz, 3H), 5.31 (s, 2H), 2.85–2.80 (m, 2H), 1.79 (dt, J = 15.4, 7.6 Hz, 2H), 1.44–1.34 (m, 2H), 0.90 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 160.72, 140.89, 134.42, 134.32, 133.94, 131.52, 129.48, 128.75, 126.24, 106.63, 47.75, 29.25, 27.49, 22.52, 13.78. MS (ESI) calculated for C17H20N3O, m/z 282.1601, found 282.1612 (M + H)+.Synthesis of compound 5: 1-benzyl-2-butyl-1H-imidazo[4,5-c]pyridin-4-amine
To a solution of compound 4 (188 mg, 0.67 mol) in 15 mL of CH2Cl2 was added benzoyl isocyanate (197 mg, 1.34 mmol) and heated at 45 °C for 2 h. The solvent was then removed under vacuum, and the residue was dissolved in 15 mL of anhydrous MeOH, followed by the addition of excess sodium methoxide. The reaction mixture was then heated at 80 °C for 1 h. The solvent was removed under vacuum and the residue was purified using column chromatography (7% MeOH–CH2Cl2) to obtain the compound 5 (56 mg, 30%). 1H NMR (500 MHz, CDCl3) δ 7.78 (d, J = 5.8 Hz, 1H), 7.34–7.28 (m, 3H), 7.03 (d, J = 6.4 Hz, 2H), 6.59 (d, J = 5.8 Hz, 1H), 5.27 (s, 2H), 5.15 (s, 2H), 2.83–2.75 (m, 2H), 1.72 (ddd, J = 13.0, 9.0, 7.7 Hz, 2H), 1.44–1.34 (m, 2H), 0.90 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 154.06, 151.00, 140.61, 140.41, 135.76, 129.21, 128.23, 126.33, 97.74, 47.52, 30.10, 27.42, 22.68, 13.89. HRMS (ESI) calculated for C17H21N4, m/z 281.1761, found 281.1795 (M + H)+.Synthesis of compound 6a: N-(1-benzyl-2-butyl-1H-imidazo[4,5-c]pyridin-4-yl)acetamide
To a solution of compound 5 (30 mg, 0.11 mmol) in 2 mL of CH2Cl2 were added triethylamine (17 μL, 0.12 mmol) and acetyl chloride (8 μL, 0.11 mmol). The reaction mixture was stirred at room temperature for 3 h and purified using column chromatography (5% MeOH–CH2Cl2) to obtain the compound 6a as white solid (6 mg, 16%). 1H NMR (500 MHz, CDCl3) δ 11.60 (s, 1H), 8.20 (s, 1H), 7.37 (dd, J = 7.7, 5.5 Hz, 3H), 7.20 (d, J = 5.6 Hz, 1H), 7.05 (dd, J = 6.3, 2.5 Hz, 2H), 5.46 (s, 2H), 3.03–2.94 (m, 2H), 2.55 (s, 3H), 1.85 (dt, J = 15.0, 7.6 Hz, 2H), 1.43 (dq, J = 14.6, 7.3 Hz, 2H), 0.92 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 141.48, 129.64, 129.40, 129.17, 126.30, 126.25, 48.47, 29.06, 24.87, 22.44, 13.60. HRMS (ESI) calculated for C19H23N4O, m/z 323.1866, found 323.1911 (M + H)+.Compounds 6b was synthesized similarly as compound 6a.
N-(1-Benzyl-2-butyl-1H-imidazo[4,5-c]pyridin-4-yl)butyramide (6b)
Butyryl chloride was used as a reagent. (4 mg, 14%). 1H NMR (500 MHz, CDCl3) δ 11.31 (s, 1H), 8.30 (d, J = 4.8 Hz, 1H), 7.38–7.34 (m, 3H), 7.27 (s, 1H), 7.04 (dd, J = 6.4, 2.6 Hz, 2H), 5.50 (s, 2H), 2.94 (t, J = 7.5 Hz, 2H), 2.78 (t, J = 7.4 Hz, 2H), 1.80 (dp, J = 22.1, 7.5 Hz, 4H), 1.41 (dt, J = 14.7, 7.4 Hz, 2H), 1.01 (t, J = 7.4 Hz, 3H), 0.91 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 174.32, 141.35, 133.50, 129.69, 129.16, 126.46, 103.38, 48.58, 39.27, 29.16, 27.45, 22.55, 18.38, 13.75. HRMS (ESI) calculated for C21H27N4O, m/z 351.2179, found 351.2240 (M + H)+.Synthesis of compound 10a: 1-benzyl-2-butyl-4-chloro-1H-imidazo[4,5-c]pyridine
4-Amino-2-chloropyridine (2.0 g, 15.6 mmol) was taken in 20 mL of conc. H2SO4 in an ice-bath to which was added 10 mL of conc. HNO3 slowly. The reaction mixture was gradually brought to room temperature and stirred for 1 h. The reaction was quenched by pouring the reaction mixture on ice. Ammonium hydroxide solution was slowly added until a pH of 3.0 was reached. A white solid was obtained which was filtered, washed with water, and dried. This (N-nitro)aminopyridine intermediate was taken up in 10 mL of conc. H2SO4 and the reaction solution was heated at 90 °C for 30 min. It was cooled to room temperature and poured into ice. It was slowly neutralized with ammonium hydroxide solution until a pH of 7 and the formed yellow solid was filtered, washed with water and dried to obtain compound 7 as a mixture of 2-chloro-3-nitropyridin-4-amine and 2-chloro-5-nitropyridin-4-amine intermediates. Sodium hydride (275 mg, 6.90 mmol) was carefully added to 20 mL of THF under N2 and compound 7 (1.0 g, 5.76 mmol) was slowly added to the solution at 0 °C. The reaction mixture was stirred for 1 h, followed by the addition of benzyl bromide (0.75 mL, 6.34 mmol). The reaction mixture was stirred at room temperature for 2 h and poured into ice water. Then it was extracted with EtOAc (3 × 20 mL), washed with water, dried over sodium sulfate. The solvent was removed and the crude residue was purified using column chromatography (20% EtOAc–hexane) to obtain the compound 8 as a mixture of regioisomeric N-benzyl-2-chloro-3-nitropyridin-4-amine and N-benzyl-2-chloro-5-nitropyridin-4-amine intermediates. To this regioisomeric mixture (1.0 g, 4.2 mmol) in 20 mL of MeOH were added zinc dust (1.4 g, 21.0 mmol) and ammonium formate (1.4 g, 21.0 mmol). The reaction mixture was stirred at room temperature for 10 min and filtered through celite. Then the solvent was evaporated and the residue was dissolved in water. This was extracted with EtOAc (3 × 20 mL), washed with water and dried over sodium sulfate. The filtrate evaporated under vacuum, and chromatographed (20% EtOAc–hexane) to obtain the required N4-benzyl-2-chloropyridine-3,4-diamine compound 9a. Also obtained was N4-benzyl-6-chloropyridine-3,4-diamine as a side-product. To a solution of compound 9a (495 mg, 2.12 mmol) in 20 mL of anhydrous THF were added triethylamine (0.31 mL, 2.23 mmol) and valeryl chloride (0.28 mL, 2.33 mmol). The reaction mixture was refluxed for 1 h. The solvent was then removed under vacuum, and the residue was dissolved in 20 mL of EtOH and NaOH (170 mg, 4.24 mmol) in 2 mL of H2O was added. The reaction mixture was refluxed for 2 h. The solvent was then removed under vacuum, and the residue was dissolved in EtOAc and washed with water. The EtOAc fraction was dried using sodium sulfate and evaporated and purified using column chromatography (5% MeOH–CH2Cl2) to obtain the compound 10a (203 mg, 32%). 1H NMR (500 MHz, CDCl3) δ 8.10 (d, J = 5.6 Hz, 1H), 7.35–7.30 (m, 3H), 7.08 (d, J = 5.6 Hz, 1H), 7.01 (dd, J = 7.4, 2.0 Hz, 2H), 5.34 (s, 2H), 2.91–2.86 (m, 2H), 1.78 (dt, J = 15.7, 7.7 Hz, 2H), 1.45–1.36 (m, 2H), 0.90 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 158.03, 141.88, 141.75, 141.12, 137.03, 134.84, 129.39, 128.58, 126.25, 105.18, 47.90, 30.04, 27.63, 22.73, 13.82. MS (ESI) calculated for C17H19ClN3, m/z 300.1262, found 300.1159 (M + H)+.Synthesis of compound 11a: 1-benzyl-N,2-dibutyl-1H-imidazo[4,5-c]pyridin-4-amine
To a solution of compound 10 (50 mg, 0.17 mmol) in 1 mL of dioxane were added potassium tert-butoxide (57 mg, 0.51 mmol), catalytic amount of 2-dicyclohexylphosphino-2′-(N,N-dimethylamino)biphenyl (DavePhos) and tris(dibenzylideneacetone)dipalladium(0) (Pd2(dba)3) and butyl amine (83 μL, 0.83 mmol). The reaction mixture was then heated under microwave conditions (500 W, 100 °C) in a sealed vial for 1 h. It was cooled to room temperature and filtered through celite and washed with MeOH. The solvent was removed and the crude residue was purified using column chromatography (5% MeOH–CH2Cl2) to obtain the compound 11a (21 mg, 36%). 1H NMR (500 MHz, CDCl3) δ 7.84 (d, J = 5.9 Hz, 1H), 7.33–7.27 (m, 3H), 7.03 (dd, J = 4.5, 3.6 Hz, 2H), 6.48 (d, J = 5.9 Hz, 1H), 5.41 (s, 1H), 5.25 (s, 2H), 3.60 (dt, J = 12.9, 6.5 Hz, 2H), 2.80–2.74 (m, 2H), 1.73–1.67 (m, 4H), 1.49 (dt, J = 15.0, 7.4 Hz, 3H), 1.38 (dd, J = 15.0, 7.5 Hz, 2H), 0.96 (t, J = 7.4 Hz, 3H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 153.15, 151.39, 135.95, 129.24, 129.15, 128.15, 127.25, 126.34, 126.18, 96.16, 47.43, 41.09, 32.22, 30.27, 27.41, 22.69, 20.47, 14.11, 13.89. HRMS (ESI) calculated for C21H29N4, m/z 337.2387, found 337.2451 (M + H)+.Compounds 11b was synthesized similarly as compound 11a.
N,1-Dibenzyl-2-butyl-1H-imidazo[4,5-c]pyridin-4-amine (11b)
Benzyl amine was used as a reagent. (33 mg, 52%). 1H NMR (500 MHz, CDCl3) δ 7.86 (d, J = 5.9 Hz, 1H), 7.45 (dd, J = 7.9, 0.9 Hz, 2H), 7.35–7.27 (m, 6H), 7.03 (d, J = 6.4 Hz, 2H), 6.54 (d, J = 5.9 Hz, 1H), 5.76 (s, 1H), 5.26 (s, 2H), 4.83 (d, J = 5.6 Hz, 2H), 2.79–2.74 (m, 2H), 1.72–1.67 (m, 2H), 1.37 (dq, J = 14.8, 7.4 Hz, 2H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 153.39, 150.92, 140.51, 139.81, 139.78, 135.88, 129.18, 128.62, 128.23, 128.19, 127.24, 126.36, 126.24, 96.73, 47.46, 45.40, 30.14, 27.38, 22.67, 13.88. HRMS (ESI) calculated for C24H27N4, m/z 371.2230, found 371.2303 (M + H)+.Synthesis of compound 11c: 1-benzyl-4-butoxy-2-butyl-1H-imidazo[4,5-c]pyridine
To a suspension of sodium hydride (48 mg, 2.00 mmol) in 2 mL of anhydrous THF was added 1-butanol (0.18 mL, 2.00 mmol). It was stirred at room temperature for 1 h, followed by the addition of compound 10 (100 mg, 0.33 mmol). The reaction mixture was heated at 60 °C for 18 h and then solvent was evaporated under vacuum. The residue was extracted with EtOAc (3 × 10 mL), washed with water and dried over sodium sulfate. The solvent was removed and the crude residue was purified using column chromatography (5% MeOH–CH2Cl2) to obtain the compound 11c (61 mg, 55%). 1H NMR (500 MHz, CDCl3) δ 7.85 (d, J = 5.8 Hz, 1H), 7.33–7.28 (m, 3H), 7.01 (dd, J = 7.7, 1.7 Hz, 2H), 6.77 (d, J = 5.8 Hz, 1H), 5.30 (s, 2H), 4.52 (t, J = 7.0 Hz, 2H), 2.87–2.82 (m, 2H), 1.90 (dd, J = 15.0, 7.1 Hz, 2H), 1.75 (dt, J = 15.7, 7.7 Hz, 2H), 1.58–1.48 (m, 2H), 1.38 (dq, J = 14.7, 7.4 Hz, 2H), 0.97 (t, J = 7.4 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 156.04, 155.18, 142.02, 138.95, 135.59, 129.21, 128.27, 127.41, 126.27, 100.47, 66.25, 47.61, 31.34, 30.21, 27.44, 22.75, 19.45, 14.09, 13.85. HRMS (ESI) calculated for C21H28N3O, m/z 338.2227, found 338.2307 (M + H)+.Synthesis of compound 12: 2,6-dichloro-3-nitropyridin-4-amine
4-Amino-2,6-dichloropyridine (2.0 g, 12.27 mmol) was added to 20 mL of conc. H2SO4. The mixture was cooled to 0 °C and 10 mL of conc. HNO3 was dropwise at 0 °C. The reaction mixture was stirred at room temperature for 1 h and then poured into crushed ice. The white solid was filtered, washed with water and dried. This intermediate was dissolved in 10 mL of conc. H2SO4 and the reaction solution was heated at 90 °C for 30 min. It was cooled to room temperature and poured into ice. It was slowly neutralized with ammonium hydroxide solution until a pH of 9 and the formed yellow solid was filtered, washed with water and dried to obtain compound 12 as light yellow solid. 1H NMR (500 MHz, MeOD) δ 6.84 (s, 1H). 13C NMR (126 MHz, MeOD) δ 152.53, 151.18, 144.57, 111.12.Synthesis of compound 13: N-benzyl-2,6-dichloro-3-nitro pyridin-4-amine
Sodium hydride (138 mg, 5.77 mmol) was carefully suspended in 10 mL of dry THF under N2. The grey suspension was cooled to 0 °C and compound 12 (1.0 g, 4.81 mmol) was slowly added to the suspension at 0 °C. The reaction mixture was stirred for 1 h, followed by the addition of benzyl bromide (0.5 mL, 5.29 mmol). The reaction mixture was stirred at room temperature for 2 h and poured into ice water. Then it was extracted with EtOAc (3 × 20 mL), washed with water, dried over sodium sulfate. The solvent was removed and the crude residue was purified using column chromatography (20% EtOAc–hexane) to obtain the compound 13 as yellow solid. 1H NMR (500 MHz, CDCl3) δ 7.44–7.34 (m, 3H), 7.32–7.27 (m, 2H), 6.99 (s, 1H), 6.67 (s, 1H), 4.47 (d, J = 5.4 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 152.07, 149.97, 145.68, 135.01, 129.48, 128.71, 127.32, 106.62, 47.74.Synthesis of compound 14: N4-benzyl-6-chloro-3-nitro-N2-(2,4,4-trimethylpentan-2-yl)pyridine-2,4-diamine
To a solution of compound 13 (300 mg, 1.02 mmol) in 20 mL of CH2Cl2 were added triethylamine (0.21 mL, 1.53 mmol) and tert-octylamine (0.5 mL, 3.06 mmol). The reaction mixture was refluxed for 48 h and the solvent was removed under vacuum. The crude residue was purified using column chromatography (20% EtOAc–hexane) to obtain the compound 14 as yellow solid (360 mg, 91%). 1H NMR (500 MHz, CDCl3) δ 9.57 (s, 1H), 9.51 (s, 1H), 7.39 (ddd, J = 7.5, 4.4, 1.3 Hz, 2H), 7.36–7.30 (m, 3H), 5.92 (s, 1H), 4.44 (d, J = 5.4 Hz, 2H), 1.95 (s, 2H), 1.56 (s, 6H), 0.98 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 155.05, 154.25, 153.44, 136.10, 129.24, 128.24, 127.43, 115.80, 94.21, 57.32, 51.54, 47.64, 31.94, 31.66, 29.84. MS (ESI) calculated for C20H28ClN4O2, m/z 391.1895, found 391.1901 (M + H)+.Synthesis of compound 16: 1-benzyl-2-butyl-6-chloro-N-(2,4,4-trimethylpentan-2-yl)-1H-imidazo[4,5-c]pyridin-4-amine
To a solution of compound 14 (260 mg, 0.66 mmol) in 20 mL of MeOH were added zinc dust (434 mg, 6.60 mmol) and ammonium formate (416 mg, 6.60 mmol). The reaction mixture was stirred at room temperature for 10 min and filtered through celite. Then the solvent was evaporated and the residue was dissolved in water. This was extracted with EtOAc (3 × 20 mL), washed with water and dried over sodium sulfate. The solvent was removed under vacuum to obtain compound 15, brown oil (184 mg, 77%). To a solution of compound 15 (184 mg, 0.50 mmol) in 10 mL of anhydrous THF were added triethylamine (74 μL, 0.52 mmol) and valeryl chloride (62 μL, 0.50 mmol). The reaction mixture was refluxed for 1 h. The solvent was then removed under vacuum, and the residue was dissolved in 10 mL of EtOH and NaOH (40 mg, 1.00 mmol) in 1 mL of H2O was added. The reaction mixture was refluxed for 5 h. The solvent was then removed under vacuum, and the residue was dissolved in EtOAc and washed with water. The EtOAc fraction was dried using sodium sulfate and evaporated and purified using column chromatography (20% EtOAc–hexane) to obtain the compound 16 as yellow solid (120 mg, 56%). 1H NMR (500 MHz, CDCl3) δ 7.34–7.28 (m, 3H), 7.02 (d, J = 6.5 Hz, 2H), 6.42 (s, 1H), 5.38 (s, 1H), 5.16 (s, 2H), 2.76–2.69 (m, 2H), 2.02 (s, 2H), 1.71–1.65 (m, 4H), 1.60 (s, 6H), 1.37 (dd, J = 15.0, 7.5 Hz, 2H), 1.01 (s, 9H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 153.37, 149.45, 141.67, 140.77, 135.64, 129.21, 128.22, 126.31, 125.50, 93.95, 55.74, 51.42, 47.42, 31.91, 31.73, 30.12, 29.96, 27.37, 22.66, 13.87. MS (ESI) calculated for C25H36ClN4, m/z 427.2623, found 427.2635 (M + H)+.Synthesis of compound 17: 1-benzyl-2-butyl-6-chloro-1H-imidazo[4,5-c]pyridin-4-amine
Compound 16 (34 mg, 0.082 mmol) was dissolved in 1 mL of HCl (4 M in dioxane) and stirred at room temperature for 30 min. Then the solvent was removed under vacuum to obtain compound 17 (11 mg, 42%). 1H NMR (500 MHz, CDCl3) δ 7.36–7.30 (m, 3H), 7.01 (d, J = 6.4 Hz, 2H), 6.58 (s, 1H), 5.23 (s, 2H), 5.21 (s, 2H), 2.80–2.73 (m, 2H), 1.72 (dt, J = 15.5, 7.7 Hz, 2H), 1.39 (dq, J = 14.8, 7.4 Hz, 2H), 0.90 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 154.83, 149.94, 142.31, 141.73, 135.26, 129.32, 128.57, 128.41, 126.26, 96.29, 47.59, 29.92, 27.36, 22.63, 13.87. HRMS (ESI) calculated for C17H20ClN4, m/z 315.1371, found 315.1422 (M + H)+.Synthesis of compound 19a: 1-benzyl-2-butyl-1H-imidazo[4,5-c]pyridine-4,6-diamine
To a solution of compound 16 (70 mg, 0.16 mmol) in 1 mL of dioxane were added potassium tert-butoxide (92 mg, 0.82 mmol), catalytic amount of DavePhos and Pd2(dba)3 and tert-octylamine (83 μL, 0.83 mmol). The reaction mixture was then heated under microwave conditions (500 W, 100 °C) in a sealed vial for 1 h. It was cooled to room temperature and filtered through celite and washed with MeOH. The solvent was removed and the crude residue was purified using column chromatography (20% EtOAc–hexane) to obtain the compound 18a, brown solid (41 mg, 49%). Compound 18a (33 mg, 0.063 mmol) was dissolved in 1 mL of HCl (4 M in dioxane) and stirred at room temperature for 30 min. Then the solvent was removed under vacuum to obtain compound 19a as brown solid (11 mg, 58%). 1H NMR (500 MHz, DMSO) δ 7.37–7.31 (m, 2H), 7.30–7.25 (m, 1H), 7.09–7.04 (m, 2H), 6.41 (s, 2H), 5.65 (s, 1H), 5.24 (s, 2H), 2.73–2.67 (m, 2H), 1.59 (dt, J = 15.3, 7.5 Hz, 2H), 1.31 (dq, J = 14.7, 7.4 Hz, 2H), 0.83 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 151.81, 147.80, 144.08, 136.91, 128.81, 128.77, 127.47, 126.29, 117.96, 76.19, 46.11, 29.27, 26.17, 21.80, 13.68. HRMS (ESI) calculated for C17H22N5, m/z 296.1870, found 296.1906 (M + H)+.Compounds 19b–19o were synthesized similarly as compound 19a.
1-Benzyl-N6,2-dibutyl-1H-imidazo[4,5-c]pyridine-4,6-diamine (19b)
Butyl amine was used as a reagent. Brown solid (23 mg, 79%). 1H NMR (500 MHz, CDCl3) δ 7.31 (ddd, J = 8.6, 6.4, 3.4 Hz, 3H), 7.05 (d, J = 6.7 Hz, 2H), 5.46 (s, 1H), 5.26 (s, 1H), 5.16 (s, 2H), 3.03 (t, J = 7.1 Hz, 2H), 2.72–2.66 (m, 2H), 1.68 (dd, J = 15.5, 7.6 Hz, 2H), 1.55 (dd, J = 14.8, 7.2 Hz, 2H), 1.43–1.33 (m, 4H), 0.90 (dt, J = 16.4, 7.4 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 152.42, 148.50, 144.42, 135.99, 129.14, 128.06, 126.33, 74.80, 47.20, 43.09, 31.41, 30.03, 27.37, 22.66, 20.40, 14.02, 13.89. HRMS (ESI) calculated for C21H30N5, m/z 352.2496, found 352.2515 (M + H)+.1-Benzyl-2-butyl-N6-heptyl-N4-(2,4,4-trimethylpentan-2-yl)-1H-imidazo[4,5-c]pyridine-4,6-diamine (18c)
Heptylamine was used as a reagent. Brown oil (43 mg, 65%). 1H NMR (500 MHz, CDCl3) δ 7.32–7.26 (m, 3H), 7.05 (d, J = 6.7 Hz, 2H), 5.39 (s, 1H), 5.13 (s, 1H), 5.11 (s, 2H), 3.13 (t, J = 7.2 Hz, 2H), 2.68–2.64 (m, 2H), 2.04 (s, 2H), 1.65–1.55 (m, 11H), 1.41–1.22 (m, 12H), 1.01 (s, 9H), 0.89–0.84 (m, 6H). 13C NMR (126 MHz, CDCl3) δ 154.28, 150.38, 149.22, 142.39, 136.63, 128.97, 128.05, 127.76, 126.40, 120.44, 95.52, 73.57, 55.27, 51.72, 47.00, 43.63, 31.97, 31.94, 31.90, 31.79, 30.47, 30.29, 29.97, 29.32, 27.44, 27.39, 22.77, 22.71, 14.25, 13.90. MS (ESI) calculated for C32H52N5, m/z 506.4217, found 506.4209 (M + H)+.1-Benzyl-2-butyl-N6-heptyl-1H-imidazo[4,5-c]pyridine-4,6-diamine (19c)
Light brown solid (24 mg, 80%). 1H NMR (500 MHz, DMSO) δ 8.03 (s, 2H), 7.38–7.33 (m, 2H), 7.32–7.28 (m, 1H), 7.12 (d, J = 7.2 Hz, 2H), 6.84 (s, 1H), 5.98 (s, 1H), 5.39 (s, 2H), 3.08 (t, J = 7.0 Hz, 2H), 2.73–2.68 (m, 2H), 1.59 (dt, J = 15.3, 8.2 Hz, 2H), 1.50 (dd, J = 14.3, 7.2 Hz, 2H), 1.33–1.21 (m, 12H), 0.85 (t, J = 7.0 Hz, 3H), 0.81 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 154.62, 145.66, 145.15, 136.32, 128.96, 128.86, 127.73, 126.64, 126.55, 74.21, 46.34, 42.24, 31.25, 28.78, 28.42, 28.09, 26.34, 26.18, 22.07, 21.71, 13.98, 13.62. HRMS (ESI) calculated for C24H36N5, m/z 394.2965, found 394.3001 (M + H)+.1-Benzyl-2-butyl-N6-(cyclohexylmethyl)-N4-(2,4,4-trimethylpentan-2-yl)-1H-imidazo[4,5-c]pyridine-4,6-diamine (18d)
N-Cyclohexylmethylamine was used as a reagent. Yellow solid (50 mg, 62%). 1H NMR (500 MHz, CDCl3) δ 7.32–7.27 (m, 3H), 7.06 (d, J = 6.7 Hz, 2H), 5.37 (s, 1H), 5.10 (s, 2H), 2.99 (d, J = 6.7 Hz, 2H), 2.69–2.64 (m, 2H), 2.04 (s, 2H), 1.79 (d, J = 13.2 Hz, 2H), 1.73–1.60 (m, 9H), 1.58 (s, 6H), 1.35 (dd, J = 15.0, 7.5 Hz, 2H), 1.27–1.13 (m, 4H), 1.01 (s, 9H), 0.98–0.91 (m, 2H), 0.86 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 154.37, 150.36, 149.19, 142.41, 136.65, 128.97, 127.76, 126.44, 120.35, 73.62, 55.26, 51.67, 50.18, 47.02, 37.88, 31.91, 31.80, 31.48, 30.51, 30.31, 27.46, 26.80, 26.17, 22.72, 13.91. MS (ESI) calculated for C32H50N5, m/z 504.4061, found 504.41 (M + H)+.1-Benzyl-2-butyl-N6-(cyclohexylmethyl)-1H-imidazo[4,5-c]pyridine-4,6-diamine (19d)
Light brown (28 mg, 80%). 1H NMR (500 MHz, DMSO) δ 7.35 (t, J = 7.3 Hz, 2H), 7.31–7.27 (m, 1H), 7.10 (d, J = 7.2 Hz, 2H), 6.34 (d, J = 3.1 Hz, 1H), 5.85 (s, 1H), 5.33 (s, 2H), 2.92 (t, J = 6.1 Hz, 2H), 2.71–2.66 (m, 2H), 1.75–1.64 (m, 4H), 1.57 (dt, J = 15.3, 7.6 Hz, 3H), 1.49 (dtd, J = 11.2, 7.4, 3.7 Hz, 1H), 1.30 (dt, J = 14.8, 7.4 Hz, 2H), 1.21–1.09 (m, 4H), 0.91 (dd, J = 22.3, 10.5 Hz, 2H), 0.81 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 153.32, 146.28, 145.05, 136.69, 128.79, 127.61, 126.53, 116.94, 74.21, 48.49, 46.22, 36.76, 30.47, 29.02, 26.21, 26.05, 25.43, 21.75, 13.65. HRMS (ESI) calculated for C24H34N5, m/z 392.2809, found 392.2806 (M + H)+.1-Benzyl-2-butyl-N6-phenyl-N4-(2,4,4-trimethylpentan-2-yl)-1H-imidazo[4,5-c]pyridine-4,6-diamine (18e)
Aniline was used as a reagent. Yellow solid (51 mg, 55%). 1H NMR (500 MHz, CDCl3) δ 7.31 (dt, J = 16.5, 5.5 Hz, 3H), 7.22 (t, J = 7.8 Hz, 2H), 7.16 (d, J = 7.6 Hz, 2H), 7.05 (d, J = 6.7 Hz, 2H), 6.90 (t, J = 6.9 Hz, 1H), 6.16 (s, 1H), 6.02 (s, 1H), 5.23 (s, 1H), 5.12 (s, 2H), 2.74–2.67 (m, 2H), 2.03 (s, 2H), 1.66 (dd, J = 15.8, 8.2 Hz, 4H), 1.60 (s, 6H), 1.38 (dd, J = 15.0, 7.5 Hz, 2H), 1.02 (s, 9H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 151.49, 149.42, 149.11, 142.46, 141.51, 136.32, 129.16, 129.06, 127.93, 126.53, 121.98, 120.91, 118.83, 55.42, 51.80, 47.26, 31.93, 31.80, 30.44, 30.19, 27.46, 22.71, 13.91. MS (ESI) calculated for C31H42N5, m/z 484.3435, found 484.3459 (M + H)+.1-Benzyl-2-butyl-N6-phenyl-1H-imidazo[4,5-c]pyridine-4,6-diamine (19e)
Light yellow solid (28 mg, 76%). 1H NMR (500 MHz, DMSO) δ 9.10 (s, 1H), 8.08 (s, 1H), 7.39–7.35 (m, 2H), 7.33–7.28 (m, 3H), 7.11 (t, J = 7.3 Hz, 4H), 7.01 (t, J = 7.3 Hz, 1H), 6.41 (s, 1H), 5.44 (s, 2H), 2.82 (t, J = 7.5 Hz, 2H), 1.63 (dt, J = 15.3, 7.6 Hz, 2H), 1.33 (dd, J = 14.9, 7.4 Hz, 2H), 0.84 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 146.34, 140.41, 136.06, 129.52, 128.92, 127.84, 126.69, 122.44, 118.87, 46.68, 28.89, 26.15, 21.72, 13.63. HRMS (ESI) calculated for C23H26N5, m/z 372.2183, found 372.2219 (M + H)+.N 6,1-Dibenzyl-2-butyl-N4-(2,4,4-trimethylpentan-2-yl)-1H-imidazo[4,5-c]pyridine-4,6-diamine (18f)
Benzyl amine was used as a reagent. Light brown solid (30 mg, 46%). 1H NMR (500 MHz, CDCl3) δ 7.35 (d, J = 7.4 Hz, 2H), 7.26 (dddd, J = 9.4, 7.2, 6.1, 1.6 Hz, 6H), 7.03 (d, J = 6.8 Hz, 2H), 5.44 (s, 1H), 5.17 (s, 1H), 5.07 (s, 2H), 4.42 (s, 2H), 2.70–2.64 (m, 2H), 1.67–1.60 (m, 2H), 1.55 (s, 6H), 1.35 (dq, J = 14.7, 7.4 Hz, 2H), 1.02–0.94 (m, 9H), 0.87 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 153.83, 150.58, 149.20, 142.27, 140.52, 136.49, 128.98, 128.56, 127.80, 127.68, 126.97, 126.46, 126.39, 74.17, 55.29, 51.62, 47.59, 47.07, 31.87, 31.76, 30.45, 30.28, 30.20, 27.42, 22.70, 13.90. MS (ESI) calculated for C32H44N5, m/z 498.3591, found 498.3599 (M + H)+.N 6,1-Dibenzyl-2-butyl-1H-imidazo[4,5-c]pyridine-4,6-diamine (19f)
White solid (22 mg, 85%). 1H NMR (500 MHz, DMSO) δ 8.07 (s, 2H), 7.38–7.35 (m, 2H), 7.34–7.29 (m, 5H), 7.29–7.25 (m, 1H), 7.07 (d, J = 6.3 Hz, 2H), 6.06 (s, 1H), 5.33 (s, 2H), 4.38 (s, 2H), 2.75–2.69 (m, 2H), 1.58 (dt, J = 15.3, 7.6 Hz, 2H), 1.30 (dt, J = 14.8, 7.4 Hz, 2H), 0.81 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 154.68, 145.29, 137.99, 136.12, 128.85, 128.45, 127.78, 127.51, 127.23, 126.74, 75.08, 46.43, 45.67, 28.76, 26.16, 21.70, 13.62. HRMS (ESI) calculated for C24H28N5, m/z 386.2339, found 386.2389 (M + H)+.1-Benzyl-2-butyl-N6-(4-methoxybenzyl)-1H-imidazo[4,5-c]pyridine-4,6-diamine (19g)
4-Methoxybenzyl amine was used as a reagent. Light yellow solid (22 mg, 69%). 1H NMR (500 MHz, DMSO) δ 8.07 (s, 2H), 7.34–7.28 (m, 5H), 7.08 (d, J = 6.4 Hz, 2H), 6.87 (d, J = 8.7 Hz, 2H), 6.04 (s, 1H), 5.34 (s, 2H), 4.29 (s, 2H), 3.72 (s, 3H), 2.75–2.70 (m, 2H), 1.58 (dt, J = 15.3, 7.6 Hz, 2H), 1.29 (dd, J = 14.9, 7.4 Hz, 2H), 0.81 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 158.48, 154.67, 145.28, 145.27, 136.13, 129.73, 128.92, 128.84, 127.78, 126.74, 113.82, 75.11, 55.08, 46.43, 45.18, 28.76, 26.16, 21.71, 13.62. HRMS (ESI) calculated for C25H30N5O, m/z 416.2445, found 416.2490 (M + H)+.1-Benzyl-2-butyl-N6-(3-methoxybenzyl)-1H-imidazo[4,5-c]pyridine-4,6-diamine (19h)
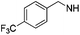
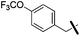
3-Methoxybenzyl amine was used as a reagent. Brown solid (27 mg, 69%). 1H NMR (500 MHz, DMSO) δ 7.32–7.27 (m, 3H), 7.23 (t, J = 7.9 Hz, 1H), 7.06 (d, J = 6.1 Hz, 2H), 6.94 (dd, J = 12.3, 4.8 Hz, 2H), 6.83 (dd, J = 8.1, 2.2 Hz, 1H), 6.03 (s, 1H), 5.31 (s, 2H), 4.33 (d, J = 5.8 Hz, 2H), 3.71 (s, 3H), 2.70 (t, J = 7.6 Hz, 2H), 1.57 (dt, J = 15.3, 7.6 Hz, 2H), 1.29 (dd, J = 14.9, 7.4 Hz, 2H), 0.81 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 159.37, 136.32, 129.52, 128.80, 127.72, 126.70, 119.55, 113.13, 112.50, 75.05, 55.00, 46.33, 45.63, 40.11, 40.02, 39.94, 28.89, 26.21, 21.72, 13.63. HRMS (ESI) calculated for C25H30N5O, m/z 416.2445, found 416.2506 (M + H)+.1-Benzyl-2-butyl-N6-(4-(trifluoromethyl)benzyl)-1H-imidazo[4,5-c]pyridine-4,6-diamine (19i)
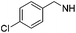
4-(Trifluoromethyl)benzyl amine was used as a reagent. Brown solid (17 mg, 77%). 1H NMR (500 MHz, DMSO) δ 7.60 (d, J = 8.2 Hz, 2H), 7.51 (d, J = 8.1 Hz, 2H), 7.29–7.21 (m, 3H), 7.02 (d, J = 6.7 Hz, 2H), 6.23 (s, 1H), 5.70 (s, 2H), 5.57 (s, 1H), 5.15 (s, 2H), 4.41 (d, J = 6.3 Hz, 2H), 2.69–2.63 (m, 2H), 1.56 (dt, J = 15.3, 7.5 Hz, 2H), 1.29 (dd, J = 14.9, 7.4 Hz, 2H), 0.82 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 153.45, 150.32, 149.41, 146.48, 143.12, 137.24, 128.58, 127.82, 127.31, 126.51, 124.88, 124.85, 118.91, 75.32, 46.04, 44.94, 29.43, 26.19, 21.82, 13.69. HRMS (ESI) calculated for C25H27F3N5, m/z 454.2213, found 454.2284 (M + H)+.1-Benzyl-2-butyl-N6-(4-chlorobenzyl)-1H-imidazo[4,5-c]pyridine-4,6-diamine (19j)
4-Chlorobenzylamine was used as a reagent. Yellow solid (15 mg, 63%). 1H NMR (500 MHz, DMSO) δ 7.33–7.25 (m, 8H), 7.02 (d, J = 6.6 Hz, 2H), 6.18 (s, 1H), 5.83 (s, 2H), 5.59 (s, 1H), 5.16 (s, 2H), 4.30 (d, J = 6.0 Hz, 2H), 2.68–2.64 (m, 2H), 1.56 (dt, J = 15.3, 7.5 Hz, 2H), 1.29 (dd, J = 14.9, 7.4 Hz, 2H), 0.82 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 150.57, 149.14, 143.25, 140.18, 137.18, 130.77, 129.08, 128.63, 127.96, 127.37, 126.56, 118.72, 75.34, 46.06, 44.71, 40.11, 40.02, 39.94, 29.40, 26.20, 21.82, 13.69. HRMS (ESI) calculated for C24H27ClN5, m/z 420.1950, found 420.1976 (M + H)+.1-Benzyl-2-butyl-N6-(furan-2-ylmethyl)-N4-(2,4,4-trimethylpentan-2-yl)-1H-imidazo[4,5-c]pyridine-4,6-diamine (18k)
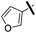
Furfuryl amine was used as a reagent. Yellow oil (53 mg, 54%). 1H NMR (500 MHz, CDCl3) δ 7.30 (ddd, J = 10.9, 4.6, 1.1 Hz, 4H), 7.04 (d, J = 6.6 Hz, 2H), 6.28 (dd, J = 3.2, 1.9 Hz, 1H), 6.15 (dd, J = 3.2, 0.6 Hz, 1H), 5.50 (s, 1H), 5.10 (s, 2H), 4.43 (s, 2H), 2.72–2.64 (m, 2H), 2.03 (s, 2H), 1.68–1.59 (m, 4H), 1.58 (s, 6H), 1.35 (dt, J = 14.7, 7.4 Hz, 2H), 1.00 (s, 9H), 0.87 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 153.92, 153.28, 150.77, 149.15, 142.19, 141.69, 136.47, 129.00, 127.83, 126.42, 110.36, 106.53, 74.85, 55.32, 51.68, 47.09, 40.78, 31.89, 31.77, 30.47, 30.31, 27.43, 22.71, 13.90. MS (ESI) calculated for C30H42N5O, m/z 488.3384, found 488.3446 (M + H)+.1-Benzyl-2-butyl-N6-(furan-2-ylmethyl)-1H-imidazo[4,5-c]pyridine-4,6-diamine (19k)
Brown solid (21 mg, 55%). 1H NMR (500 MHz, DMSO) δ 7.55 (s, 1H), 7.34 (t, J = 7.3 Hz, 2H), 7.31–7.27 (m, 1H), 7.10 (d, J = 7.3 Hz, 2H), 6.36 (dd, J = 3.0, 1.9 Hz, 1H), 6.29 (d, J = 2.6 Hz, 1H), 5.99 (s, 1H), 5.31 (s, 2H), 4.35 (d, J = 5.8 Hz, 2H), 2.73–2.67 (m, 2H), 1.58 (dt, J = 15.2, 7.6 Hz, 2H), 1.30 (dd, J = 14.9, 7.4 Hz, 2H), 0.82 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 142.17, 136.66, 128.79, 127.62, 126.61, 110.39, 107.43, 75.34, 60.19, 46.26, 38.71, 29.07, 26.21, 21.76, 13.65. HRMS (ESI) calculated for C22H26N5O, m/z 376.2132, found 376.2184 (M + H)+.1-Benzyl-2-butyl-N6-(pyridin-4-ylmethyl)-N4-(2,4,4-trimethyl pentan-2-yl)-1H-imidazo[4,5-c]pyridine-4,6-diamine (18l)
4-Picolylamine was used as a reagent. Yellow solid (25 mg, 31%). 1H NMR (500 MHz, CDCl3) δ 8.48 (dd, J = 4.5, 1.6 Hz, 2H), 7.29–7.25 (m, 3H), 7.24 (dd, J = 4.5, 1.5 Hz, 2H), 7.01 (d, J = 6.1 Hz, 2H), 5.40 (s, 1H), 5.06 (s, 2H), 4.49 (s, 2H), 2.70–2.64 (m, 2H), 1.92 (s, 2H), 1.63 (dt, J = 15.5, 7.7 Hz, 2H), 1.48 (s, 6H), 1.36 (dt, J = 14.9, 7.4 Hz, 2H), 0.95 (s, 9H), 0.87 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 149.83, 136.31, 129.01, 127.89, 126.40, 122.20, 74.67, 55.25, 51.56, 47.13, 46.09, 31.81, 31.75, 31.71, 30.44, 30.23, 27.41, 22.69, 13.89. MS (ESI) calculated for C31H43N6, m/z 499.3544, found 499.3475 (M + H)+.1-Benzyl-2-butyl-N6-(pyridin-4-ylmethyl)-1H-imidazo[4,5-c]pyridine-4,6-diamine (19l)
Brown solid (7 mg, 47%). 1H NMR (500 MHz, DMSO) δ 8.43 (dd, J = 4.5, 1.5 Hz, 2H), 7.27 (ddd, J = 9.7, 6.8, 4.4 Hz, 5H), 7.03 (d, J = 6.7 Hz, 2H), 6.50–6.28 (m, 2H), 5.65 (s, 1H), 5.18 (s, 2H), 4.37 (d, J = 6.3 Hz, 2H), 2.69–2.65 (m, 2H), 1.57 (dt, J = 15.3, 7.5 Hz, 2H), 1.30 (dd, J = 14.9, 7.4 Hz, 2H), 0.81 (d, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 149.28, 137.09, 130.51, 128.65, 127.43, 126.53, 122.35, 113.97, 75.37, 46.09, 44.38, 29.34, 26.19, 21.80, 13.68. HRMS (ESI) calculated for C23H27N6, m/z 387.2292, found 387.2299 (M + H)+.1-Benzyl-2-butyl-N6-(pyridin-3-ylmethyl)-1H-imidazo[4,5-c]pyridine-4,6-diamine (19m)
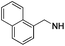
3-Picolylamine was used as a reagent. Light brown solid (33 mg, 63%). 1H NMR (500 MHz, DMSO) δ 8.92 (s, 1H), 8.80 (d, J = 5.3 Hz, 1H), 8.46 (d, J = 8.0 Hz, 1H), 8.12 (s, 2H), 7.94 (dd, J = 7.9, 5.7 Hz, 1H), 7.69 (s, 1H), 7.33–7.25 (m, 3H), 7.04 (d, J = 6.9 Hz, 2H), 6.03 (s, 1H), 5.32 (s, 2H), 4.64 (s, 2H), 2.72 (t, J = 7.6 Hz, 2H), 1.56 (dt, J = 15.2, 7.6 Hz, 2H), 1.28 (dd, J = 14.9, 7.4 Hz, 2H), 0.80 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 154.65, 145.80, 145.01, 143.55, 141.29, 141.12, 138.17, 136.10, 128.83, 127.76, 126.68, 126.52, 75.49, 46.45, 42.51, 28.77, 26.12, 21.68, 13.60. HRMS (ESI) calculated for C23H27N6, m/z 387.2292, found 387.2296 (M + H)+.1-Benzyl-2-butyl-N6-(naphthalen-1-ylmethyl)-1H-imidazo[4,5-c]pyridine-4,6-diamine (19n)
1-Naphthylmethylamine was used as a reagent. Light brown solid (28 mg, 70%). 1H NMR (500 MHz, DMSO) δ 12.12 (s, 1H), 8.10 (d, J = 8.0 Hz, 1H), 8.04 (s, 2H), 7.99–7.96 (m, 1H), 7.88 (d, J = 8.2 Hz, 1H), 7.61–7.53 (m, 3H), 7.45 (dd, J = 8.1, 7.1 Hz, 1H), 7.33–7.28 (m, 3H), 7.08 (d, J = 6.4 Hz, 2H), 6.22 (s, 1H), 5.35 (s, 2H), 4.83 (s, 2H), 2.74–2.68 (m, 2H), 1.58 (dt, J = 15.3, 7.6 Hz, 2H), 1.29 (dd, J = 14.9, 7.4 Hz, 2H), 0.81 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 154.69, 145.51, 145.31, 136.23, 133.44, 132.90, 130.92, 128.83, 128.63, 127.99, 127.76, 126.67, 126.39, 125.97, 125.49, 125.43, 123.63, 74.91, 46.39, 43.99, 28.80, 26.20, 21.71, 13.62. HRMS (ESI) calculated for C28H30N5, m/z 436.2496, found 436.2549 (M + H)+.N 6-([1,1′-Biphenyl]-4-ylmethyl)-1-benzyl-2-butyl-1H-imidazo[4,5-c]pyridine-4,6-diamine (19o)
[1,1′-biphenyl]-4-yl methanamine was used as a reagent. Light brown solid (21 mg, 51%). 1H NMR (500 MHz, DMSO) δ 12.36 (s, 1H), 8.04 (s, 2H), 7.63 (dd, J = 12.4, 7.8 Hz, 4H), 7.50–7.42 (m, 4H), 7.40–7.33 (m, 1H), 7.28 (t, J = 7.3 Hz, 2H), 7.23 (t, J = 7.2 Hz, 1H), 7.07 (d, J = 7.3 Hz, 2H), 6.07 (s, 1H), 5.33 (s, 2H), 4.42 (s, 2H), 2.71 (t, J = 7.6 Hz, 2H), 1.57 (dt, J = 15.2, 7.7 Hz, 2H), 1.29 (dq, J = 14.6, 7.3 Hz, 2H), 0.81 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 154.65, 145.40, 145.35, 139.80, 139.07, 137.24, 136.17, 128.95, 128.80, 128.06, 127.67, 127.43, 126.71, 126.69, 126.57, 75.11, 46.38, 45.31, 28.81, 26.18, 21.69, 13.61. HRMS (ESI) calculated for C30H32N5, m/z 462.2652, found 462.2698 (M + H)+.Synthesis of compound 19p and 19r: N6-(4-(aminomethyl)benzyl)-1-benzyl-2-butyl-1H-imidazo[4,5-c]pyridine-4,6-diamine
To a solution of compound 16 (70 mg, 0.16 mmol) in 1 mL of dioxane were added potassium tert-butoxide (90 mg, 0.80 mmol), catalytic amount of DavePhos and Pd2(dba)3 and p-xylylenediamine (109 mg, 0.80 mmol). The reaction mixture was then heated under microwave conditions (500 W, 100 °C) in a sealed vial for 1 h. It was cooled to room temperature and filtered through celite and washed with MeOH. The solvent was removed and the crude residue was purified using column chromatography (20% EtOAc–hexane) to obtain the compound 18p and 18r. Compound 18p (33 mg, 0.063 mmol) was dissolved in 1 mL of HCl (4 M in dioxane) and stirred at room temperature for 30 min. Then the solvent was removed under vacuum to obtain compound 19p as light yellow solid (5 mg, 63%). 1H NMR (500 MHz, DMSO) δ 8.37 (s, 2H), 8.06 (s, 2H), 7.42 (q, J = 8.5 Hz, 4H), 7.36–7.30 (m, 3H), 7.08 (d, J = 6.8 Hz, 2H), 6.08 (s, 1H), 5.34 (s, 2H), 4.40 (s, 2H), 3.98 (q, J = 5.8 Hz, 2H), 2.75–2.67 (m, 2H), 1.57 (dt, J = 15.3, 7.6 Hz, 2H), 1.28 (dd, J = 14.9, 7.4 Hz, 2H), 0.80 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 154.71, 145.41, 145.34, 138.43, 136.19, 132.98, 129.02, 128.89, 127.79, 127.75, 126.65, 46.40, 45.31, 41.89, 28.78, 26.18, 21.70, 13.62. HRMS (ESI) calculated for C25H31N6, m/z 415.2605, found 415.2606 (M + H)+.N 6,N6′-(1,4-Phenylenebis(methylene))bis(1-benzyl-2-butyl-1H-imidazo[4,5-c]pyridine-4,6-diamine) (19r)
Compound 18r (35 mg, 0.038 mmol) was dissolved in 1 mL of HCl (4 M in dioxane) and stirred at room temperature for 30 min. Then the solvent was removed under vacuum to obtain compound 19r as light yellow solid (12 mg, 45%).1H NMR (500 MHz, MeOD) δ 7.27 (s, 4H), 7.21–7.17 (m, 6H), 7.02–6.98 (m, 4H), 5.20 (s, 4H), 4.33 (s, 4H), 2.78–2.73 (m, 4H), 1.67 (dt, J = 15.3, 7.6 Hz, 4H), 1.37 (dd, J = 15.0, 7.5 Hz, 4H), 0.89 (t, J = 7.4 Hz, 6H). 13C NMR (126 MHz, MeOD) δ 155.94, 150.96, 148.16, 146.47, 138.77, 137.12, 130.00, 129.03, 128.70, 127.70, 118.63, 48.01, 47.26, 30.28, 27.76, 23.29, 14.05. HRMS (ESI) calculated for C42H49N10, m/z 693.4136, found 693.4331 (M + H)+.Compounds 19q and 19s were synthesized similarly as compounds 19p and 19r.
N 6-(3-(Aminomethyl)benzyl)-1-benzyl-2-butyl-1H-imidazo[4,5-c]pyridine-4,6-diamine (19q)
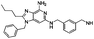
m-Xylylenediamine was used as a reagent. Light yellow solid. 1H NMR (500 MHz, MeOD) δ 7.49 (s, 1H), 7.41 (dd, J = 3.9, 1.5 Hz, 2H), 7.39–7.35 (m, 1H), 7.32–7.28 (m, 3H), 7.05–7.02 (m, 2H), 5.31 (s, 2H), 4.44 (s, 2H), 4.07 (s, 2H), 2.79–2.75 (m, 2H), 1.70 (dt, J = 15.3, 7.6 Hz, 2H), 1.38 (dq, J = 14.8, 7.4 Hz, 2H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 157.36, 147.23, 146.79, 140.00, 136.86, 134.96, 130.63, 130.12, 129.26, 129.19, 129.14, 129.00, 127.62, 48.10, 47.15, 44.18, 30.00, 27.75, 23.24, 14.03. HRMS (ESI) calculated for C25H31N6, m/z 415.2605, found 415.2610 (M + H)+.N 6,N6′-(1,3-Phenylenebis(methylene))bis(1-benzyl-2-butyl-1H-imidazo[4,5-c]pyridine-4,6-diamine) (19s)
Light yellow solid. 1H NMR (500 MHz, DMSO) δ 12.38 (s, 2H), 8.04 (s, 4H), 7.38 (s, 1H), 7.30–7.22 (m, 9H), 7.04 (d, J = 6.8 Hz, 4H), 6.01 (s, 2H), 5.28 (s, 4H), 4.34 (s, 4H), 2.73–2.68 (m, 4H), 1.56 (dd, J = 15.3, 7.7 Hz, 4H), 1.29 (dd, J = 14.9, 7.4 Hz, 4H), 0.81 (t, J = 7.4 Hz, 6H). 13C NMR (126 MHz, DMSO) δ 154.62, 145.36, 145.31, 138.22, 136.15, 128.80, 128.61, 127.72, 126.66, 126.56, 126.38, 46.36, 45.73, 28.80, 26.19, 21.71, 13.62. HRMS (ESI) calculated for C42H49N10, m/z 693.4136, found 693.4131 (M + H)+.Synthesis of compound 23a
To a solution of compound 14 (120 mg, 0.31 mmol) in 1 mL of dioxane were added cesium carbonate (303 mg, 0.93 mmol) in H2O (0.5 mL), [1,1′-bis(diphenylphosphino)ferrocene]dichloropalladium(II) (Pd(dppf)Cl2) (15 mg, 0.019 mmol) and n-butylboronic acid (98 μL, 0.46 mmol) under N2. The reaction mixture was then heated at 90 °C in a sealed vial for 18 h. It was cooled to room temperature and filtered through celite and washed with MeOH. The solvent was removed and the crude residue was purified using column chromatography (15% EtOAc–hexane) to obtain the compound 20a (97 mg, 76%). To a solution of compound 20a (94 mg, 0.23 mmol) in 10 mL of MeOH were added zinc dust (149 mg, 2.30 mmol) and ammonium formate (145 mg, 2.30 mmol). The reaction mixture was stirred at room temperature for 10 min and filtered through celite. Then the solvent was evaporated and the residue was dissolved in water. This was extracted with EtOAc (3 × 20 mL), washed with water and dried over sodium sulfate. The solvent was removed under vacuum to obtain compound 21a (45 mg, 51%). To a solution of compound 21a (42 mg, 0.11 mmol) in 7 mL of anhydrous THF were added triethylamine (16 μL, 0.12 mmol) and valeryl chloride (13 μL, 0.11 mmol). The reaction mixture was refluxed for 1 h. The solvent was then removed under vacuum, and the residue was dissolved in 5 mL of EtOH and NaOH (10 mg, 0.22 mmol) in 1 mL of H2O was added. The reaction mixture was refluxed for 18 h. The solvent was then removed under vacuum, and the residue was dissolved in EtOAc and washed with water. The EtOAc fraction was dried using sodium sulfate and evaporated and purified using column chromatography (20% EtOAc–hexane) to obtain the compound 22a (25 mg, 51%). Compound 22a (22 mg, 0.049 mmol) was dissolved in 1 mL of HCl (4 M in dioxane) and stirred at room temperature for 30 min. Then the solvent was removed under vacuum to obtain compound 23a (11 mg, 69%).N 4-Benzyl-6-butyl-3-nitro-N2-(2,4,4-trimethylpentan-2-yl)pyridine-2,4-diamine (20a)
Yellow oil (97 mg, 76%). 1H NMR (500 MHz, CDCl3) δ 9.51 (t, J = 4.4 Hz, 1H), 9.40 (s, 1H), 7.37 (ddd, J = 7.1, 4.4, 1.6 Hz, 2H), 7.34–7.30 (m, 3H), 5.74 (s, 1H), 4.45 (d, J = 5.4 Hz, 2H), 2.44 (t, J = 7.5 Hz, 2H), 2.01 (s, 2H), 1.67–1.61 (m, 2H), 1.30 (dd, J = 15.0, 7.4 Hz, 2H), 0.96 (s, 9H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 166.49, 154.69, 152.58, 136.94, 129.07, 127.92, 127.42, 115.70, 94.41, 56.59, 51.17, 47.44, 38.87, 31.95, 31.64, 30.61, 30.19, 22.43, 14.11. MS (ESI) calculated for C24H37N4O2, m/z 413.2911, found 413.3267 (M + H)+.1-Benzyl-2,6-dibutyl-1H-imidazo[4,5-c]pyridin-4-amine (23a)
White solid (11 mg, 69%). 1H NMR (500 MHz, CDCl3) δ 7.37–7.31 (m, 3H), 7.10 (d, J = 6.9 Hz, 2H), 6.81 (s, 1H), 5.47 (s, 2H), 2.85 (t, 2H), 2.72 (t, 2H), 1.74 (dd, J = 15.3, 7.6 Hz, 2H), 1.67 (ddd, J = 15.3, 10.4, 7.0 Hz, 2H), 1.38 (ddq, J = 14.8, 10.2, 7.4 Hz, 4H), 0.94 (t, J = 7.4 Hz, 3H), 0.90 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 157.75, 150.13, 143.74, 137.10, 130.16, 129.22, 127.59, 124.62, 97.63, 35.61, 32.84, 30.27, 27.85, 23.31, 23.22, 14.12, 14.04. HRMS (ESI) calculated for C21H29N4, m/z 337.2387, found 337.2476 (M + H)+.Compounds 23b–23j were synthesized similarly as compound 23a.
N 4-Benzyl-3-nitro-6-phenyl-N2-(2,4,4-trimethylpentan-2-yl)pyridine-2,4-diamine (20b)
Phenylboronic acid was used as a reagent. Yellow solid (28 mg, 57%). 1H NMR (500 MHz, CDCl3) δ 9.68 (s, 1H), 9.54 (s, 1H), 7.92 (ddd, J = 4.4, 2.5, 1.4 Hz, 2H), 7.45–7.42 (m, 3H), 7.40–7.38 (m, 3H), 7.34–7.31 (m, 1H), 7.26 (s, 1H), 6.39 (s, 1H), 4.58 (d, J = 5.4 Hz, 2H), 2.09 (s, 2H), 1.64 (s, 6H), 0.99 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 159.01, 154.74, 153.13, 138.88, 136.87, 130.20, 129.19, 128.66, 128.06, 127.50, 127.44, 92.16, 56.72, 51.69, 47.62, 32.02, 31.74, 30.15. MS (ESI) calculated for C26H33N4O2, m/z 433.2598, found 433.2615 (M + H)+.1-Benzyl-2-butyl-6-phenyl-N-(2,4,4-trimethylpentan-2-yl)-1H-imidazo[4,5-c]pyridin-4-amine (22b)
Yellow solid (31 mg, 47%). 1H NMR (500 MHz, CDCl3) δ 8.02 (dd, J = 8.3, 1.2 Hz, 2H), 7.39 (t, J = 7.7 Hz, 2H), 7.34–7.27 (m, 4H), 7.07 (d, J = 6.7 Hz, 2H), 6.94 (s, 1H), 5.34 (s, 1H), 5.28 (s, 2H), 2.79–2.73 (m, 2H), 2.18 (s, 2H), 1.73–1.66 (m, 8H), 1.39 (dd, J = 15.0, 7.5 Hz, 2H), 1.03 (s, 9H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 153.22, 150.01, 148.43, 141.28, 140.32, 136.16, 129.61, 129.42, 129.15, 128.41, 128.07, 127.67, 127.54, 126.72, 126.36, 126.28, 126.22, 91.77, 55.45, 51.63, 47.28, 31.98, 31.81, 30.33, 30.25, 27.50, 22.72, 13.90. MS (ESI) calculated for C31H41N4, m/z 469.3326, found 469.3350 (M + H)+.1-Benzyl-2-butyl-6-phenyl-1H-imidazo[4,5-c]pyridin-4-amine (23b)
White solid (13 mg, 73%). 1H NMR (500 MHz, CDCl3) δ 7.91 (d, J = 7.2 Hz, 2H), 7.54–7.44 (m, 3H), 7.42–7.34 (m, 3H), 7.05 (dd, J = 6.4, 1.1 Hz, 2H), 6.83 (s, 1H), 5.37 (s, 2H), 2.88–2.78 (m, 2H), 1.78 (dd, J = 15.2, 7.8 Hz, 2H), 1.42 (dq, J = 14.7, 7.4 Hz, 2H), 0.93 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 157.54, 149.18, 142.11, 141.93, 134.08, 132.07, 130.82, 129.68, 129.66, 129.01, 127.38, 126.25, 95.25, 48.13, 29.31, 27.40, 22.52, 13.84. HRMS (ESI) calculated for C23H25N4, m/z 357.2074, found 357.2155 (M + H)+.4-(4-(Benzylamino)-5-nitro-6-((2,4,4-trimethylpentan-2-yl)amino)pyridin-2-yl)benzonitrile (20c)
4-Cyanophenylboronic acid was used as a reagent. Yellow solid (30 mg, 55%). 1H NMR (500 MHz, CDCl3) δ 9.72 (s, 1H), 9.51 (s, 1H), 7.97 (d, J = 8.6 Hz, 2H), 7.72 (d, J = 8.6 Hz, 2H), 7.44–7.31 (m, 5H), 6.38 (s, 1H), 4.59 (d, J = 5.4 Hz, 2H), 2.06 (s, 2H), 1.63 (s, 6H), 0.99 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 156.75, 154.69, 153.26, 143.14, 136.53, 132.50, 129.28, 128.23, 127.93, 127.35, 118.79, 113.38, 93.05, 56.87, 51.72, 47.68, 32.01, 31.72, 30.10. MS (ESI) calculated for C27H32N5O2, m/z 458.2551, found 458.2571 (M + H)+.4-(1-Benzyl-2-butyl-4-((2,4,4-trimethylpentan-2-yl)amino)-1H-imidazo[4,5-c]pyridin-6-yl)benzamide (22c)
Cyano group was converted into amide in the basic condition. Yellow solid (22 mg, 41%). 1H NMR (500 MHz, CDCl3) δ 8.10 (d, J = 8.5 Hz, 2H), 7.84 (d, J = 8.5 Hz, 2H), 7.34–7.28 (m, 3H), 7.08–7.04 (m, 2H), 6.99 (s, 1H), 6.14 (s, 1H), 5.79 (s, 1H), 5.41 (s, 1H), 5.29 (s, 2H), 2.81–2.74 (m, 2H), 2.17 (s, 2H), 1.72–1.66 (m, 8H), 1.43–1.33 (m, 2H), 1.03 (s, 9H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 169.46, 153.74, 150.08, 147.00, 144.87, 140.11, 135.97, 131.83, 129.20, 128.15, 127.62, 126.76, 126.74, 126.31, 92.63, 55.50, 51.55, 47.31, 31.95, 31.79, 30.25, 30.17, 27.46, 22.69, 13.89. MS (ESI) calculated for C32H42N5O, m/z 512.3384, found 512.3408 (M + H)+.4-(4-Amino-1-benzyl-2-butyl-1H-imidazo[4,5-c]pyridin-6-yl)benzamide (23c)
White solid (11 mg, 79%). 1H NMR (500 MHz, MeOD) δ 8.09–8.05 (m, 2H), 7.89–7.85 (m, 2H), 7.51 (s, 1H), 7.41–7.32 (m, 3H), 7.17 (d, J = 7.0 Hz, 2H), 5.62 (s, 2H), 3.76–3.72 (m, 1H), 3.68–3.64 (m, 1H), 3.58 (dd, J = 7.0, 2.7 Hz, 1H), 2.94–2.89 (m, 2H), 1.78 (dt, J = 15.3, 7.6 Hz, 2H), 1.42 (dd, J = 15.0, 7.5 Hz, 2H), 0.92 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 171.03, 159.91, 149.79, 143.90, 141.45, 137.23, 136.75, 136.73, 130.29, 129.74, 129.43, 128.43, 127.76, 125.80, 98.94, 73.57, 72.45, 62.18, 43.73, 30.01, 27.99, 23.28, 14.04. HRMS (ESI) calculated for C24H26N5O, m/z 400.2132, found 400.2177 (M + H)+.N 4-Benzyl-5-nitro-N6-(2,4,4-trimethylpentan-2-yl)-[2,3′-bipyridine]-4,6-diamine (20d)
4-Pyridinylboronic acid was used as a reagent. Orange solid (135 mg, 82%). 1H NMR (500 MHz, CDCl3) δ 9.74 (t, J = 5.1 Hz, 1H), 9.54 (s, 1H), 9.10 (dd, J = 2.2, 0.6 Hz, 1H), 8.65 (dd, J = 4.8, 1.6 Hz, 1H), 8.21–8.16 (m, 1H), 7.42–7.36 (m, 5H), 7.35–7.31 (m, 1H), 6.38 (s, 1H), 4.59 (d, J = 5.5 Hz, 2H), 2.07 (s, 2H), 1.63 (s, 6H), 0.99 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 156.52, 154.78, 153.22, 150.86, 148.93, 136.60, 134.75, 134.36, 129.26, 128.17, 127.35, 123.54, 116.37, 92.38, 56.86, 51.65, 47.65, 32.01, 31.72, 30.11. MS (ESI) calculated for C25H32N5O2, m/z 434.2551, found 434.2563 (M + H)+.1-Benzyl-2-butyl-6-(pyridin-3-yl)-1H-imidazo[4,5-c]pyridin-4-amine (23d)
Light yellow solid (10 mg, 72%). 1H NMR (500 MHz, MeOD) δ 9.28 (d, J = 1.9 Hz, 1H), 8.94 (dd, J = 5.5, 1.2 Hz, 1H), 8.83 (d, J = 8.2 Hz, 1H), 8.11 (ddd, J = 8.2, 5.6, 0.5 Hz, 1H), 7.72 (s, 1H), 7.40–7.32 (m, 3H), 7.18 (d, J = 6.9 Hz, 2H), 5.65 (s, 2H), 2.98–2.91 (m, 2H), 1.78 (dd, J = 15.3, 7.7 Hz, 2H), 1.42 (dd, J = 15.0, 7.5 Hz, 2H), 0.92 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 150.19, 143.97, 143.57, 143.11, 136.48, 130.41, 130.32, 129.52, 128.03, 127.74, 127.73, 100.47, 48.55, 29.93, 27.90, 23.26, 14.02. HRMS (ESI) calculated for C22H24N5, m/z 358.2026, found 358.2067 (M + H)+.N 4-Benzyl-6-(furan-3-yl)-3-nitro-N2-(2,4,4-trimethylpentan-2-yl)pyridine-2,4-diamine (20e)
3-Furylboronic acid was used as a reagent. Yellow solid (95 mg, 70%). 1H NMR (500 MHz, CDCl3) δ 9.68 (t, J = 4.8 Hz, 1H), 9.49 (s, 1H), 7.95 (dd, J = 1.5, 0.8 Hz, 1H), 7.45 (t, J = 1.7 Hz, 1H), 7.41–7.34 (m, 4H), 7.34–7.30 (m, 1H), 6.69 (dd, J = 1.9, 0.8 Hz, 1H), 6.07 (s, 1H), 4.53 (d, J = 5.4 Hz, 2H), 2.05 (s, 2H), 1.60 (s, 6H), 0.98 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 154.88, 153.66, 153.09, 143.99, 143.68, 136.85, 129.19, 128.06, 127.39, 127.36, 108.83, 91.66, 56.68, 51.51, 47.61, 31.99, 31.71, 30.13. MS (ESI) calculated for C24H31N4O3, m/z 423.2391, found 423.2401 (M + H)+.1-Benzyl-2-butyl-6-(furan-3-yl)-N-(2,4,4-trimethylpentan-2-yl)-1H-imidazo[4,5-c]pyridin-4-amine (22e)
Brown oil (30 mg, 41%). 1H NMR (500 MHz, CDCl3) δ 7.94 (dd, J = 1.6, 0.7 Hz, 1H), 7.41 (t, J = 1.7 Hz, 1H), 7.34–7.27 (m, 3H), 7.05 (d, J = 6.6 Hz, 2H), 6.76 (dd, J = 1.8, 0.8 Hz, 1H), 6.61 (s, 1H), 5.30 (s, 1H), 5.24 (s, 2H), 2.76–2.71 (m, 2H), 2.13 (s, 2H), 1.70–1.65 (m, 2H), 1.64 (s, 6H), 1.36 (dt, J = 14.7, 7.4 Hz, 2H), 1.02 (s, 9H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 152.80, 150.15, 143.24, 142.70, 140.71, 140.01, 136.16, 129.15, 128.83, 128.06, 126.33, 125.88, 108.87, 91.20, 55.44, 51.45, 47.25, 31.93, 31.79, 30.29, 30.16, 27.45, 22.71, 13.90. MS (ESI) calculated for C29H39N4O, m/z 459.3118, found 459.3168 (M + H)+.1-Benzyl-2-butyl-6-(furan-3-yl)-1H-imidazo[4,5-c]pyridin-4-amine (23e)
Light yellow solid (12 mg, 64%). 1H NMR (500 MHz, MeOD) δ 8.23–8.19 (m, 1H), 7.73–7.70 (m, 1H), 7.42 (s, 1H), 7.40–7.32 (m, 3H), 7.15 (d, J = 6.9 Hz, 2H), 6.97 (dd, J = 2.0, 0.9 Hz, 1H), 5.58 (s, 2H), 2.90–2.85 (m, 2H), 1.75 (dt, J = 15.3, 7.6 Hz, 2H), 1.40 (dq, J = 14.8, 7.4 Hz, 2H), 0.91 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 159.50, 149.42, 146.46, 144.24, 142.92, 136.72, 135.38, 130.27, 129.41, 127.69, 124.89, 121.45, 109.21, 96.82, 48.27, 29.95, 27.91, 23.26, 14.03. HRMS (ESI) calculated for C21H23N4O, m/z 347.1866, found 347.1921 (M + H)+.N 4-Benzyl-3-nitro-6-(thiophen-3-yl)-N2-(2,4,4-trimethylpentan-2-yl)pyridine-2,4-diamine (20f)
3-Thienylboronic acid was used as a reagent. Yellow solid (107 mg, 76%). 1H NMR (500 MHz, CDCl3) δ 9.69 (s, 1H), 9.52 (s, 1H), 7.89 (dd, J = 3.0, 1.2 Hz, 1H), 7.49 (dd, J = 5.1, 1.2 Hz, 1H), 7.42–7.30 (m, 6H), 6.26 (s, 1H), 4.56 (d, J = 5.4 Hz, 2H), 2.08 (s, 2H), 1.63 (s, 6H), 0.99 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 154.88, 154.61, 153.25, 142.21, 136.90, 129.19, 128.05, 127.38, 126.59, 126.56, 126.28, 91.99, 56.68, 51.59, 47.61, 32.02, 31.72, 30.16. MS (ESI) calculated for C24H31N4O2S, m/z 439.2162, found 439.2183 (M + H)+.1-Benzyl-2-butyl-6-(thiophen-3-yl)-N-(2,4,4-trimethylpentan-2-yl)-1H-imidazo[4,5-c]pyridin-4-amine (22f)
Brown solid (25 mg, 35%). 1H NMR (500 MHz, CDCl3) δ 7.79 (dd, J = 3.1, 1.2 Hz, 1H), 7.58 (dd, J = 5.0, 1.2 Hz, 1H), 7.33–7.27 (m, 4H), 7.06 (d, J = 6.6 Hz, 2H), 6.79 (s, 1H), 5.35 (s, 1H), 5.25 (s, 2H), 2.78–2.70 (m, 2H), 2.17 (s, 2H), 1.70 (dd, J = 8.7, 7.0 Hz, 3H), 1.67 (s, 6H), 1.38 (dq, J = 14.7, 7.4 Hz, 2H), 1.26 (dd, J = 8.7, 5.6 Hz, 1H), 1.02 (s, 9H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 153.04, 150.02, 144.89, 144.32, 140.11, 136.13, 129.14, 128.07, 126.45, 126.34, 125.94, 125.41, 121.55, 91.54, 55.43, 51.49, 50.96, 47.26, 31.96, 31.80, 30.26, 30.21, 27.46, 22.71, 13.89. MS (ESI) calculated for C29H39N4S, m/z 475.2890, found 475.2922 (M + H)+.1-Benzyl-2-butyl-6-(thiophen-3-yl)-1H-imidazo[4,5-c]pyridin-4-amine (23f)
White solid (9 mg, 53%). 1H NMR (500 MHz, MeOD) δ 8.04 (dd, J = 2.9, 1.4 Hz, 1H), 7.66 (dd, J = 5.1, 2.9 Hz, 1H), 7.58 (dd, J = 5.1, 1.4 Hz, 1H), 7.48 (s, 1H), 7.41–7.32 (m, 3H), 7.17 (d, J = 6.9 Hz, 2H), 5.59 (s, 2H), 2.94–2.86 (m, 2H), 1.76 (dt, J = 15.3, 7.6 Hz, 2H), 1.41 (dq, J = 14.8, 7.4 Hz, 2H), 0.91 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 159.63, 149.38, 144.24, 137.90, 136.73, 135.24, 130.28, 129.42, 129.31, 127.74, 126.73, 126.03, 124.88, 97.21, 49.07, 29.96, 27.94, 23.26, 14.03. HRMS (ESI) calculated for C21H23N4S, m/z 363.1638, found 363.1686 (M + H)+.N 4,6-Dibenzyl-3-nitro-N2-(2,4,4-trimethylpentan-2-yl)pyridine-2,4-diamine (20g)
Benzylboronic acid pinacol ester was used as a reagent. Orange oil (135 mg, 91%). 1H NMR (500 MHz, CDCl3) δ 9.54 (t, J = 5.0 Hz, 1H), 9.37 (s, 1H), 7.36 (ddd, J = 7.4, 4.5, 1.4 Hz, 2H), 7.31 (dt, J = 9.8, 4.4 Hz, 1H), 7.27 (ddd, J = 6.7, 3.3, 1.4 Hz, 5H), 7.22–7.18 (m, 3H), 5.75 (s, 1H), 4.40 (d, J = 5.4 Hz, 2H), 3.75 (s, 2H), 1.89 (s, 2H), 1.49 (s, 6H), 0.90 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 164.70, 154.68, 152.84, 138.77, 136.80, 129.45, 129.07, 128.54, 127.91, 127.46, 126.50, 124.96, 115.72, 94.56, 56.63, 51.05, 47.44, 45.61, 31.87, 31.60, 30.09, 24.86. MS (ESI) calculated for C27H35N4O2, m/z 447.2755, found 447.2737 (M + H)+.1,6-Dibenzyl-2-butyl-N-(2,4,4-trimethylpentan-2-yl)-1H-imidazo[4,5-c]pyridin-4-amine (22g)
Light brown solid (55 mg, 60%). 1H NMR (500 MHz, CDCl3) δ 7.33–7.27 (m, 5H), 7.24 (t, J = 7.6 Hz, 2H), 7.14 (t, J = 7.3 Hz, 1H), 7.01 (d, J = 6.4 Hz, 2H), 6.31 (s, 1H), 5.22 (s, 1H), 5.16 (s, 2H), 3.96 (s, 2H), 2.74–2.67 (m, 2H), 2.02 (s, 2H), 1.66–1.61 (m, 2H), 1.56 (s, 6H), 1.35 (dd, J = 15.0, 7.5 Hz, 2H), 0.93 (s, 9H), 0.86 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 152.37, 151.90, 150.06, 141.57, 140.10, 136.28, 129.31, 129.06, 128.21, 127.95, 126.38, 125.78, 124.94, 93.59, 55.35, 50.98, 47.19, 45.39, 31.85, 31.68, 30.41, 30.27, 27.42, 22.67, 13.87. MS (ESI) calculated for C32H43N4, m/z 483.3482, found 483.3526 (M + H)+.1,6-Dibenzyl-2-butyl-1H-imidazo[4,5-c]pyridin-4-amine (23g)
White solid (23 mg, 58%). 1H NMR (500 MHz, DMSO) δ 13.63 (s, 1H), 8.27 (s, 2H), 7.37–7.29 (m, 7H), 7.27–7.23 (m, 1H), 7.15 (s, 1H), 7.11 (d, J = 6.9 Hz, 2H), 5.52 (s, 2H), 4.08 (s, 2H), 2.86–2.79 (m, 2H), 1.61 (dt, J = 15.3, 7.6 Hz, 2H), 1.31 (dt, J = 14.9, 7.4 Hz, 2H), 0.81 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 156.72, 148.03, 142.12, 141.72, 137.39, 135.93, 128.96, 128.72, 128.70, 127.92, 126.98, 126.63, 123.29, 97.60, 46.84, 40.11, 38.20, 28.89, 26.28, 21.69, 13.61. HRMS (ESI) calculated for C24H27N4, m/z 371.2230, found 371.2285 (M + H)+.N 4-Benzyl-6-(4-methylbenzyl)-3-nitro-N2-(2,4,4-trimethylpentan-2-yl)pyridine-2,4-diamine (20h)
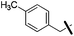
4-Methyl benzylboronic acid pinacol ester was used as a reagent. Yellow oil (130 mg, 91%). 1H NMR (500 MHz, CDCl3) δ 9.52 (t, J = 5.0 Hz, 1H), 9.37 (s, 1H), 7.37–7.30 (m, 3H), 7.28–7.24 (m, 3H), 7.10–7.03 (m, 7H), 5.74 (s, 1H), 4.39 (d, J = 5.4 Hz, 2H), 3.70 (s, 2H), 2.31 (s, 3H), 1.90 (s, 2H), 1.51 (s, 6H), 0.90 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 165.00, 154.68, 152.83, 136.82, 135.98, 135.70, 135.50, 134.26, 129.28, 129.23, 129.12, 129.05, 128.98, 127.89, 127.47, 115.70, 94.47, 83.50, 56.63, 51.03, 47.44, 45.22, 31.87, 31.57, 30.12, 24.87, 21.19, 21.11. MS (ESI) calculated for C28H37N4O2, m/z 461.2911, found 461.3003 (M + H)+.1-Benzyl-2-butyl-6-(4-methylbenzyl)-1H-imidazo[4,5-c]pyridin-4-amine (23h)
White solid (19 mg, 66%). 1H NMR (500 MHz, MeOD) δ 7.38–7.32 (m, 3H), 7.16 (d, J = 7.9 Hz, 2H), 7.13–7.08 (m, 4H), 6.89 (s, 1H), 5.49 (s, 2H), 4.08 (s, 2H), 2.92–2.87 (m, 2H), 2.32 (s, 3H), 1.76 (dt, J = 15.3, 7.6 Hz, 2H), 1.40 (dt, J = 14.8, 7.4 Hz, 2H), 0.91 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 159.11, 149.35, 144.27, 143.93, 138.37, 136.53, 134.52, 130.68, 130.25, 129.85, 129.42, 127.74, 124.41, 99.41, 39.40, 30.01, 27.84, 23.26, 21.08, 14.03. HRMS (ESI) calculated for C25H29N4, m/z 385.2387, found 385.2451 (M + H)+.N 4-Benzyl-3-nitro-6-(4-(trifluoromethoxy)benzyl)-N2-(2,4,4-trimethylpentan-2-yl)pyridine-2,4-diamine (20i)
4-(Trifluoromethoxy)benzylboronic acid pinacol ester was used as a reagent. Yellow solid (115 mg, 70%). 1H NMR (500 MHz, CDCl3) δ 9.57 (t, J = 5.0 Hz, 1H), 9.37 (s, 1H), 7.38–7.29 (m, 3H), 7.28 (t, J = 1.8 Hz, 2H), 7.21–7.17 (m, 2H), 7.10 (d, J = 7.9 Hz, 2H), 5.74 (s, 1H), 4.43 (d, J = 5.5 Hz, 2H), 1.83 (s, 2H), 1.45 (s, 6H), 0.88 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 163.87, 154.70, 152.91, 137.54, 136.71, 130.77, 129.10, 127.95, 127.33, 121.09, 115.75, 94.57, 56.63, 50.96, 47.44, 44.69, 31.82, 31.55, 30.02. MS (ESI) calculated for C28H34F3N4O3, m/z 531.2578, found 531.2738 (M + H)+.1-Benzyl-2-butyl-6-(4-(trifluoromethoxy)benzyl)-1H-imidazo[4,5-c]pyridin-4-amine (23i)
White solid (21 mg, 47%). 1H NMR (500 MHz, CDCl3) δ 7.37–7.29 (m, 5H), 7.21 (d, J = 8.0 Hz, 2H), 7.08 (d, J = 6.5 Hz, 2H), 6.79 (s, 1H), 5.45 (s, 2H), 4.09 (s, 2H), 2.87 (dd, J = 9.3, 6.1 Hz, 2H), 1.74 (dt, J = 15.3, 7.6 Hz, 2H), 1.41 (dt, J = 15.0, 7.4 Hz, 2H), 0.91 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 157.96, 150.50, 149.43, 149.42, 143.48, 138.54, 136.96, 131.59, 130.16, 129.25, 127.66, 124.97, 122.37, 99.02, 40.76, 30.29, 27.86, 23.31, 14.05. HRMS (ESI) calculated for C25H26F3N4O, m/z 455.2053, found 455.2131 (M + H)+.N 4-Benzyl-3-nitro-6-phenethyl-N2-(2,4,4-trimethylpentan-2-yl)pyridine-2,4-diamine (20j)
2-Phenylethylboronic acid was used as a reagent. Yellow oil (85 mg, 59%). 1H NMR (500 MHz, CDCl3) δ 9.52 (t, J = 4.9 Hz, 1H), 9.43 (s, 1H), 7.36 (t, J = 7.2 Hz, 2H), 7.33–7.28 (m, 4H), 7.27–7.25 (m, 3H), 7.19 (d, J = 7.3 Hz, 1H), 7.17 (d, J = 7.0 Hz, 2H), 5.69 (s, 1H), 4.39 (d, J = 5.4 Hz, 2H), 2.99 (dd, J = 9.1, 6.8 Hz, 2H), 2.75 (dd, J = 9.1, 6.8 Hz, 2H), 2.04 (s, 2H), 1.58 (s, 6H), 0.98 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 165.06, 154.74, 152.65, 141.77, 136.85, 129.09, 128.52, 128.49, 127.95, 127.39, 126.10, 115.78, 94.52, 56.68, 51.35, 47.41, 40.87, 34.61, 31.97, 31.69, 30.20. MS (ESI) calculated for C28H37N4O2, m/z 461.2911, found 461.3096 (M + H)+.1-Benzyl-2-butyl-6-phenethyl-1H-imidazo[4,5-c]pyridin-4-amine (23j)
Yellow solid (11 mg, 42%). 1H NMR (500 MHz, CDCl3) δ 7.36–7.30 (m, 3H), 7.23–7.18 (m, 2H), 7.13 (ddd, J = 5.7, 3.7, 1.5 Hz, 3H), 7.02 (d, J = 6.5 Hz, 2H), 6.68 (s, 1H), 5.38 (s, 2H), 2.99 (s, 4H), 2.85–2.79 (m, 2H), 1.69 (ddd, J = 13.2, 8.5, 6.7 Hz, 2H), 1.37 (dq, J = 14.8, 7.4 Hz, 2H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 157.35, 150.48, 148.64, 143.39, 141.89, 137.14, 130.14, 129.53, 129.43, 129.16, 127.59, 127.22, 124.68, 97.93, 48.17, 38.42, 36.84, 30.28, 27.83, 23.31, 14.03. HRMS (ESI) calculated for C25H29N4, m/z 385.2387, found 385.2446 (M + H)+.Synthesis of compound 24: 3-nitrobenzo[g]quinolin-4-ol
Nitromethane (0.96 mL, 18 mmol) was added dropwise to a solution of NaOH (2.2 g, 54 mmol) in water (5 mL), at 0 °C. The mixture was then warmed to 40 °C and nitromethane (0.96 mL, 18 mmol) was again added slowly at 40–45 °C. The temperature was maintained until a clear solution was obtained. The reaction mixture was then heated to 55 °C for 2–5 minutes, cooled to 30 °C, poured onto crushed ice and acidified with conc. HCl (5 mL). The resultant solution of methazoic acid was added immediately to a filtered solution of 3-amino-2-naphtholic acid (3 g, 16 mmol) and conc. HCl (1 mL) in water (20 mL). The reaction mixture was allowed to stand at room temperature for 12 h. After filtration, the residue obtained was washed with water, and dried (1.1 g, 90%). A solution of intermediate (2 g, 7.75 mmol) in acetic anhydride (10 mL) was placed in a 2-neck flask fitted with a reflux condenser. It was stirred and heated to 105 °C until a clear solution was obtained. Heating was then discontinued and potassium acetate (0.77 g, 7.90 mmol) was added. The mixture was then refluxed for 15 min with vigorous stirring, until a solid started to precipitate. The reaction mixture was then slowly cooled to room temperature. The residue was filtered, washed with glacial acetic acid until the washings were colorless, then suspended in water, filtered, washed with water and dried at 110 °C to get compound 24 (0.93 g, 50%). 1H NMR (500 MHz, DMSO) δ 13.12 (s, 1H), 9.28 (s, 1H), 8.95 (s, 1H), 8.27–8.21 (m, 2H), 8.11 (d, J = 8.4 Hz, 1H), 7.69 (t, J = 7.1 Hz, 1H), 7.61 (t, J = 7.1 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 168.61, 144.40, 135.05, 134.63, 130.14, 129.54, 128.94, 128.71, 127.46, 127.41, 126.65, 126.48, 116.78. MS (ESI) calculated for C13H8N2O3, m/z 240.05, found 263.04 (M + Na)+.Synthesis of compound 25: 4-chloro-3-nitrobenzo[g]quinoline
A suspension of compound 24 (2.0 g, 8.30 mmol) in phosphorus(V) oxychloride was placed in a pressure vessel and it was heated at 150 °C. After a clear solution was obtained, the reaction mixture was kept at 150 °C for 1 h. Then it was slowly cooled to room temperature and the solvent was evaporated under vacuum. The residue was poured over crushed ice while stirring and the formed solid was filtered, washed with water and dried to obtain compound 25 (1.95 g, 91%). 1H NMR (500 MHz, DMSO) δ 13.34 (d, J = 6.9 Hz, 1H), 9.26 (d, J = 7.3 Hz, 1H), 8.95 (s, 1H), 8.25 (d, J = 9.4 Hz, 2H), 8.11 (d, J = 8.4 Hz, 1H), 7.72–7.67 (m, 1H), 7.61 (ddd, J = 8.1, 6.8, 1.1 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 168.64, 144.23, 134.96, 134.64, 130.16, 129.56, 128.98, 128.70, 127.48, 127.43, 126.69, 126.48, 116.74.Synthesis of compound 26: N-benzyl-3-nitrobenzo[g]quinolin-4-amine
To a solution of compound 25 (1.0 g, 3.90 mol) in 20 mL of CH2Cl2 was added triethylamine (0.81 mL, 5.80 mmol) and benzylamine (0.50 mL, 4.60 mmol). The reaction mixture was refluxed for 2 h. The solvent was then evaporated under vacuum and H2O was added to the residue. The solution was extracted with CH2Cl2 (3 × 20 mL), washed with water and dried over sodium sulfate. The solvent was evaporated and the residue was purified using silica gel column chromatography (5% MeOH–CH2Cl2) to obtain compound 26 as a yellow solid (1.1 g, 88%). 1H NMR (500 MHz, CDCl3) δ 10.57 (s, 1H), 9.41 (s, 1H), 8.87 (s, 1H), 8.50 (s, 1H), 8.02 (d, J = 8.4 Hz, 1H), 7.83 (d, J = 8.4 Hz, 1H), 7.65–7.60 (m, 1H), 7.55–7.51 (m, 1H), 7.50–7.47 (m, 4H), 7.42 (ddd, J = 11.0, 5.4, 3.0 Hz, 1H), 5.28 (d, J = 5.9 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 152.50, 147.73, 145.88, 136.85, 135.48, 130.53, 129.64, 129.12, 129.03, 128.98, 128.83, 128.11, 128.04, 127.29, 126.86, 124.21, 118.09, 53.19. MS (ESI) calculated for C20H16N3O2, m/z 330.1237, found 330.1304 (M + H)+.Synthesis of compound 30: 1-benzyl-2-butyl-1H-benzo[g]imidazo[4,5-c]quinolin-4-amine
To a solution of compound 26 (300 mg, 0.91 mmol) in 20 mL of MeOH were added zinc dust (594 mg, 9.10 mmol) and ammonium formate (574 mg, 9.10 mmol). The reaction mixture was stirred at room temperature for 30 min and filtered through celite. Then the solvent was evaporated and the residue was dissolved in water. This was extracted with EtOAc (3 × 20 mL), washed with water and dried over sodium sulfate. The solvent was removed under vacuum to obtain compound 27 (100 mg, 37%). To a solution of compound 27 (98 mg, 0.33 mmol) in 10 mL of anhydrous THF were added triethylamine (48 μL, 0.35 mmol) and valeryl chloride (40 μL, 0.33 mmol). The reaction mixture was refluxed for 2 h. The solvent was then removed under vacuum, and the residue was dissolved in 10 mL of EtOH and NaOH (26 mg, 0.66 mmol) in 1 mL of H2O was added. The reaction mixture was refluxed for 2 h. The solvent was then removed under vacuum, and the residue was dissolved in EtOAc and washed with water. The EtOAc fraction was dried using sodium sulfate and evaporated and purified using column chromatography (10% MeOH–CH2Cl2) to obtain the compound 28 (76 mg, 63%). To a solution of compound 28 (76 mg, 0.21 mmol) in a solvent mixture of MeOH–CH2Cl2–CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) was added 3-chloroperoxy benzoic acid (443 mg, 1.98 mmol), and the solution was refluxed at 45–50 °C for 1 h. The solvent was then removed and the residue was purified using column chromatography (10% MeOH–CH2Cl2) to obtain the N-oxide derivative 29 (64 mg, 80%). To a solution of compound 29 (64 mg, 0.17 mol) in 10 mL of CH2Cl2 was added benzoyl isocyanate (37 mg, 0.25 mmol) and heated at 45 °C for 18 h. The solvent was then removed under vacuum, and the residue was dissolved in 15 mL of anhydrous MeOH, followed by the addition of excess sodium methoxide. The reaction mixture was then heated at 80 °C for 2 h. The solvent was removed under vacuum and the residue was purified using column chromatography (10% MeOH–CH2Cl2) to obtain the compound 30 (20 mg, 30%).1H NMR (500 MHz, DMSO) δ 8.40 (s, 1H), 8.05 (s, 1H), 7.88 (d, J = 8.3 Hz, 1H), 7.77 (d, J = 8.3 Hz, 1H), 7.38 (d, J = 7.2 Hz, 1H), 7.33 (dd, J = 12.9, 5.2 Hz, 3H), 7.24 (d, J = 7.4 Hz, 1H), 7.16 (d, J = 7.4 Hz, 2H), 6.91 (s, 2H), 6.02 (s, 2H), 3.00–2.92 (m, 2H), 1.74 (dt, J = 15.4, 7.6 Hz, 2H), 1.40 (dd, J = 14.9, 7.4 Hz, 2H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 136.73, 131.80, 128.91, 127.99, 127.81, 127.46, 126.82, 125.81, 123.63, 118.72, 48.20, 29.71, 26.27, 21.85, 13.69. HRMS (ESI) calculated for C25H25N4, m/z 381.2074, found 381.2089 (M + H)+.
10) was added 3-chloroperoxy benzoic acid (443 mg, 1.98 mmol), and the solution was refluxed at 45–50 °C for 1 h. The solvent was then removed and the residue was purified using column chromatography (10% MeOH–CH2Cl2) to obtain the N-oxide derivative 29 (64 mg, 80%). To a solution of compound 29 (64 mg, 0.17 mol) in 10 mL of CH2Cl2 was added benzoyl isocyanate (37 mg, 0.25 mmol) and heated at 45 °C for 18 h. The solvent was then removed under vacuum, and the residue was dissolved in 15 mL of anhydrous MeOH, followed by the addition of excess sodium methoxide. The reaction mixture was then heated at 80 °C for 2 h. The solvent was removed under vacuum and the residue was purified using column chromatography (10% MeOH–CH2Cl2) to obtain the compound 30 (20 mg, 30%).1H NMR (500 MHz, DMSO) δ 8.40 (s, 1H), 8.05 (s, 1H), 7.88 (d, J = 8.3 Hz, 1H), 7.77 (d, J = 8.3 Hz, 1H), 7.38 (d, J = 7.2 Hz, 1H), 7.33 (dd, J = 12.9, 5.2 Hz, 3H), 7.24 (d, J = 7.4 Hz, 1H), 7.16 (d, J = 7.4 Hz, 2H), 6.91 (s, 2H), 6.02 (s, 2H), 3.00–2.92 (m, 2H), 1.74 (dt, J = 15.4, 7.6 Hz, 2H), 1.40 (dd, J = 14.9, 7.4 Hz, 2H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 136.73, 131.80, 128.91, 127.99, 127.81, 127.46, 126.82, 125.81, 123.63, 118.72, 48.20, 29.71, 26.27, 21.85, 13.69. HRMS (ESI) calculated for C25H25N4, m/z 381.2074, found 381.2089 (M + H)+.
Human TLR-7/-8 reporter gene assays (NF-κB induction)
The induction of NF-κB was quantified using HEK-Blue-7 (hTLR7-specific) and HEK-Blue-8 (hTLR8-specific) cells as previously described by us.33,45,46 HEK293 cells stably co-transfected with human TLR7 or human TLR8, MD2, and secreted alkaline phosphatase (sAP), were maintained in HEK-Blue™ Selection medium containing zeocin and normocin. Stable expression of secreted alkaline phosphatase (sAP) under control of NF-κB/AP-1 promoters is inducible by appropriate TLR agonists, and extracellular sAP in the supernatant is proportional to NF-κB induction. HEK-Blue cells were incubated at a density of ∼105 cells per mL in a volume of 80 µL per well, in 384-well, flat-bottomed, cell culture-treated microtiter plates until confluency was achieved, and subsequently stimulated with graded concentrations of stimuli. sAP was assayed spectrophotometrically using an alkaline phosphatase-specific chromogen (present in HEK-detection medium as supplied by the vendor) at 620 nm.Immunoassays for interferon (IFN)-α, and cytokines
Fresh human peripheral blood mononuclear cells (hPBMC) were isolated from human blood obtained by venipuncture with informed consent and as per institutional guidelines on Ficoll-Hypaque gradients as described elsewhere.64 Aliquots of PBMCs (105 cells in 100 μL per well) were stimulated for 12 h with graded concentrations of test compounds. Supernatants were isolated by centrifugation, and were assayed in triplicates using either high-sensitivity multi-subtype IFN-α ELISA kits (PBL Interferon Source, Piscataway, NJ and R&D Systems, Inc., Minneapolis, MN), or analyte-specific multiplexed cytokine/chemokine bead array assays as reported by us previously.65Flow-cytometric immunostimulation experiments
CD69 upregulation was determined by flow cytometry using protocols published by us previously,33 and modified for rapid-throughput. Briefly, heparin-anticoagulated whole blood samples were obtained by venipuncture from healthy human volunteers with informed consent and as per guidelines approved by the University of Kansas Human Subjects Experimentation Committee. Serial dilutions of selected imidazopyridine compounds (and imiquimod, used as a reference compound) were performed using a Bio-Tek Precision 2000 XS liquid handler in sterile 96-well polypropylene plates, to which were added 100 μL aliquots of anticoagulated whole human blood. The plates were incubated at 37 °C for 16.5 h. Negative (endotoxin free water) controls were included in each experiment. Following incubation, fluorochrome-conjugated antibodies (CD3-PE, CD56-APC, CD69-PE-Cy7, 10 μL of each antibody, Becton-Dickinson Biosciences, San Jose, CA) were added to each well with a liquid handler, and incubated at 37 °C in the dark for 30 min. Following staining, erythrocytes were lysed and leukocytes fixed by mixing 200 mL of the samples in 2 mL pre-warmed Whole Blood Lyse/Fix Buffer (Becton-Dickinson Biosciences, San Jose, CA) in 96 deep-well plates. After washing the cells twice at 200 g for 8 minutes in saline, the cells were transferred to a 96-well plate. Flow cytometry was performed using a BD FACSArray instrument in the tri-color mode (tri-color flow experiment) and two-color mode (two-color flow experiment) for acquisition on 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 gated events. Compensation for spillover was computed for each experiment on singly-stained samples. CD69 activation in the major lymphocytic populations, viz., natural killer lymphocytes (NK cells: CD3−CD56+), cytokine-induced killer phenotype (CIK cells: CD3+CD56+), nominal B lymphocytes (CD3−CD56−), and nominal T lymphocytes (CD3+CD56−) were quantified using FlowJo v 7.0 software (Treestar, Ashland, OR).
000 gated events. Compensation for spillover was computed for each experiment on singly-stained samples. CD69 activation in the major lymphocytic populations, viz., natural killer lymphocytes (NK cells: CD3−CD56+), cytokine-induced killer phenotype (CIK cells: CD3+CD56+), nominal B lymphocytes (CD3−CD56−), and nominal T lymphocytes (CD3+CD56−) were quantified using FlowJo v 7.0 software (Treestar, Ashland, OR).
Acknowledgements
This work was supported by NIH/NIAID contract HSN272200900033C.Notes and references
- A. Iwasaki and R. Medzhitov, Science, 2010, 327, 291 CrossRef CAS PubMed.
- A. Iwasaki and R. Medzhitov, Nat. Immunol., 2004, 5, 987 CrossRef CAS PubMed.
- C. A. Janeway Jr. and R. Medzhitov, Annu. Rev. Immunol., 2002, 20, 197 CrossRef PubMed.
- M. S. Lee and Y. J. Kim, Mol. Cells, 2007, 23, 1 CAS.
- B. A. Seaton, E. C. Crouch, F. X. McCormack, J. F. Head, K. L. Hartshorn and R. Mendelsohn, Innate Immun., 2010, 16, 143 CrossRef CAS PubMed.
- V. L. Runza, W. Schwaeble and D. N. Mannel, Immunobiology, 2008, 213, 297 CrossRef CAS PubMed.
- A. Agrawal, P. P. Singh, B. Bottazzi, C. Garlanda and A. Mantovani, Adv. Exp. Med. Biol., 2009, 653, 98 CrossRef CAS PubMed.
- M. E. Bianchi, J. Leukocyte Biol., 2007, 81, 1 CrossRef CAS PubMed.
- M. Yoneyama and T. Fujita, J. Biol. Chem., 2007, 282, 15315 CrossRef CAS PubMed.
- M. H. Shaw, T. Reimer, Y. G. Kim and G. Nunez, Curr. Opin. Immunol., 2008, 20, 377 CrossRef CAS PubMed.
- T. Kawai and S. Akira, Nat. Immunol., 2010, 11, 373 CrossRef CAS PubMed.
- A. Iwasaki and R. Medzhitov, Nat. Immunol., 2004, 5, 987 CrossRef CAS PubMed.
- Y. Kumagai, O. Takeuchi and S. Akira, J. Infect. Chemother., 2008, 14, 86 CrossRef CAS PubMed.
- Y. Kumagai, O. Takeuchi and S. Akira, J. Infect. Chemother., 2008, 14, 86 CrossRef CAS PubMed.
- K. Takeda and S. Akira, Curr. Protoc. Immunol., 2007 DOI:10.1002/0471142735.im1412s77.
- S. Akira, Adv. Immunol., 1902, 78, 1 Search PubMed.
- S. Akira, K. Takeda and T. Kaisho, Nat. Immunol., 2001, 2, 675 CrossRef CAS PubMed.
- A. Cottalorda, C. Verschelde, A. Marcais, M. Tomkowiak, P. Musette, S. Uematsu, S. Akira, J. Marvel and N. Bonnefoy-Berard, Eur. J. Immunol., 2006, 36, 1684 CrossRef CAS PubMed.
- T. Kaisho and S. Akira, Biochim. Biophys. Acta, 2002, 1589, 1 CrossRef CAS.
- G. R. Stark, I. M. Kerr, B. R. Williams, R. H. Silverman and R. D. Schreiber, Annu. Rev. Biochem., 1998, 67, 227 CrossRef CAS PubMed.
- S. S. Diebold, T. Kaisho, H. Hemmi, S. Akira and C. Reis e Sousa, Science, 2004, 303, 1529 CrossRef CAS PubMed.
- A. D. Edwards, S. S. Diebold, E. M. Slack, H. Tomizawa, H. Hemmi, T. Kaisho, S. Akira and C. Reis e Sousa, Eur. J. Immunol., 2003, 33, 827 CrossRef CAS PubMed.
- D. Rajagopal, C. Paturel, Y. Morel, S. Uematsu, S. Akira and S. S. Diebold, Blood, 2010, 115, 1949 CrossRef CAS PubMed.
- A. Krug, G. D. Luker, W. Barchet, D. A. Leib, S. Akira and M. Colonna, Blood, 2004, 103, 1433 CrossRef CAS PubMed.
- J. Lund, A. Sato, S. Akira, R. Medzhitov and A. Iwasaki, J. Exp. Med., 2003, 198, 513 CrossRef CAS PubMed.
- A. ISAACS and J. Lindemann, Proc. R. Soc. London, Ser. B, 1957, 147, 258 CrossRef CAS.
- A. Le Bon, N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow and D. F. Tough, Nat. Immunol., 2003, 4, 1009 CrossRef CAS PubMed.
- A. Le Bon, G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli and D. F. Tough, Immunity, 2001, 14, 461 CrossRef CAS PubMed.
- D. Durali, M. G. de Goer de Herve, J. Giron-Michel, B. Azzarone, J. F. Delfraissy and Y. Taoufik, Blood, 2003, 102, 4084 CrossRef CAS PubMed.
- F. E. Lund and T. D. Randall, Nat. Rev. Immunol., 2010, 10, 236 CrossRef CAS PubMed.
- M. G. de Goer de Herve, D. Durali, B. Dembele, M. Giuliani, T. A. Tran, B. Azzarone, P. Eid, M. Tardieu, J. F. Delfraissy and Y. Taoufik, PLoS One, 2011, 6, e19366 CAS.
- T. Sareneva, S. Matikainen, M. Kurimoto and I. Julkunen, J. Immunol., 1998, 160, 6032 CAS.
- J. D. Hood, H. J. Warshakoon, M. R. Kimbrell, N. M. Shukla, S. Malladi, X. Wang and S. A. David, Hum. Vaccin., 2010, 6, 1 CrossRef.
- N. M. Shukla, T. C. Lewis, T. P. Day, C. A. Mutz, R. Ukani, C. D. Hamilton, R. Balakrishna and S. A. David, Bioorg. Med. Chem. Lett., 2011, 21, 3232 CrossRef CAS PubMed.
- N. J. Horscroft, D. C. Pryde and H. Bright, J. Antimicrob. Chemother., 2012, 67, 789 CrossRef CAS PubMed.
- S. J. Bergman, M. C. Ferguson and C. Santanello, Infect. Dis. Clin. North Am., 2011, 25, 819 CrossRef PubMed.
- B. J. Weigel, S. Cooley, T. Defor, D. J. Weisdorf, A. Panoskaltsis-Mortari, W. Chen, B. R. Blazar and J. S. Miller, Am. J. Hematol., 2012, 953 CrossRef CAS PubMed.
- J. F. Gerster, K. J. Lindstrom, R. L. Miller, M. A. Tomai, W. Birmachu, S. N. Bomersine, S. J. Gibson, L. M. Imbertson, J. R. Jacobson, R. T. Knafla, P. V. Maye, N. Nikolaides, F. Y. Oneyemi, G. J. Parkhurst, S. E. Pecore, M. J. Reiter, L. S. Scribner, T. L. Testerman, N. J. Thompson, T. L. Wagner, C. E. Weeks, J. D. Andre, D. Lagain, Y. Bastard and M. Lupu, J. Med. Chem., 2005, 48, 3481 CrossRef CAS PubMed.
- K. Hirota, K. Kazaoka, I. Niimoto, H. Kumihara, H. Sajiki, Y. Isobe, H. Takaku, M. Tobe, H. Ogita, T. Ogino, S. Ichii, A. Kurimoto and H. Kawakami, J. Med. Chem., 2002, 45, 5419 CrossRef CAS PubMed.
- Y. Isobe, M. Tobe, H. Ogita, A. Kurimoto, T. Ogino, H. Kawakami, H. Takaku, H. Sajiki, K. Hirota and H. Hayashi, Bioorg. Med. Chem., 2003, 11, 3641 CrossRef CAS PubMed.
- A. Kurimoto, T. Ogino, S. Ichii, Y. Isobe, M. Tobe, H. Ogita, H. Takaku, H. Sajiki, K. Hirota and H. Kawakami, Bioorg. Med. Chem., 2004, 12, 1091 CrossRef CAS PubMed.
- A. Kurimoto, K. Hashimoto, T. Nakamura, K. Norimura, H. Ogita, H. Takaku, R. Bonnert, T. McInally, H. Wada and Y. Isobe, J. Med. Chem., 2010, 53, 2964 CrossRef CAS PubMed.
- J. Lee, T. H. Chuang, V. Redecke, L. She, P. M. Pitha, D. A. Carson, E. Raz and H. B. Cottam, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 6646 CrossRef CAS PubMed.
- A. X. Xiang, S. E. Webber, B. M. Kerr, E. J. Rueden, J. R. Lennox, G. J. Haley, T. Wang, J. S. Ng, M. R. Herbert, D. L. Clark, V. N. Banh, W. Li, S. P. Fletcher, K. R. Steffy, D. M. Bartkowski, L. I. Kirkovsky, L. A. Bauman and D. R. Averett, Nucleosides Nucleotides Nucleic Acids, 2007, 26, 635 CAS.
- N. M. Shukla, M. R. Kimbrell, S. S. Malladi and S. A. David, Bioorg. Med. Chem. Lett., 2009, 19, 2211 CrossRef CAS PubMed.
- N. M. Shukla, S. S. Malladi, C. A. Mutz, R. Balakrishna and S. A. David, J. Med. Chem., 2010, 53, 4450 CrossRef CAS PubMed.
- N. M. Shukla, S. S. Malladi, V. Day and S. A. David, Bioorg. Med. Chem., 2011, 19, 3801 CrossRef CAS PubMed.
- N. M. Shukla, C. A. Mutz, R. Ukani, H. J. Warshakoon, D. S. Moore and S. A. David, Bioorg. Med. Chem. Lett., 2010, 20, 6384 CrossRef CAS PubMed.
- N. M. Shukla, C. A. Mutz, S. S. Malladi, H. J. Warshakoon, R. Balakrishna and S. A. David, J. Med. Chem., 2012, 55, 1106 CrossRef CAS PubMed.
- E. Brummer, M. A. Antonysamy, L. Bythadka, G. W. Gullikson and D. A. Stevens, FEMS Immunol. Med. Microbiol., 2010, 59, 81 CrossRef CAS PubMed.
- K. J. Lindstrom and N. Nikolaides, Imidazo[4,5-c]pyridin-4-amines, World patent, WO 95/02597, 1995 Search PubMed.
- T. A. Kshirsagar, B. A. Merril, S. E. Langer, K. J. Lindstrom, S. C. Johannessen, G. J. Marszalek, J. R. Wurst, K. J. Manske, S. Niwas, G. D. Lundquist Jr., P. D. Heppner, G. W. Griesgraber and M. E. Danielson, Method of preferentially inducing the biosynthesis of interferon, World patent, WO 2006/091467 A2[1], 2006, 252 Search PubMed.
- T. A. Kshirsagar, G. D. Lundquist Jr.J. F. Dellaria Jr.M. R. Radmer and B. M. Zimmermann, Oxime and hydroxylamine substituted imidazo[4,5-c] ring compounds and methods, World patent, WO 2006/086634, 2006 Search PubMed.
- J. F. Dellaria, G. A. Haraldson, P. D. Heppner, K. J. Lindstrom and B. A. Merrill, Sulfonamido substituted imidazopyridines, US6,525,064 B1, 2003, 1–35 Search PubMed.
- J. D. Hood, H. J. Warshakoon, M. R. Kimbrell, N. M. Shukla, S. Malladi, X. Wang and S. A. David, Hum. Vaccin., 2010, 6, 1 CrossRef.
- A. T. Baviskar, C. Madaan, R. Preet, P. Mohapatra, V. Jain, A. Agarwal, S. K. Guchhait, C. N. Kundu, U. C. Banerjee and P. V. Bharatam, J. Med. Chem., 2011, 54, 5013 CrossRef CAS PubMed.
- M. S. Jin and J. O. Lee, Curr. Opin. Immunol., 2008, 20, 414 CrossRef CAS PubMed.
- S. I. Yoon, O. Kurnasov, V. Natarajan, M. Hong, A. V. Gudkov, A. L. Osterman and I. A. Wilson, Science, 2012, 335, 859 CrossRef CAS PubMed.
- H. Hemmi, T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda and S. Akira, Nat. Immunol., 2002, 3, 196 CrossRef CAS PubMed.
- T. Kaisho and S. Akira, J. Allergy Clin. Immunol., 2006, 117, 979 CrossRef CAS PubMed.
- K. B. Gorden, K. S. Gorski, S. J. Gibson, R. M. Kedl, W. C. Kieper, X. Qiu, M. A. Tomai, S. S. Alkan and J. P. Vasilakos, J. Immunol., 2005, 174, 1259 CrossRef CAS.
- K. S. Gorski, E. L. Waller, J. Bjornton-Severson, J. A. Hanten, C. L. Riter, W. C. Kieper, K. B. Gorden, J. S. Miller, J. P. Vasilakos, M. A. Tomai and S. S. Alkan, Int. Immunol., 2006, 18, 1115 CrossRef CAS PubMed.
- H. P. Kokatla, E. Yoo, D. B. Salunke, D. Sil, C. F. Ng, R. Balakrishna, S. S. Malladi, L. M. Fox and S. A. David, Org. Biomol. Chem., 2013, 11, 1179 CAS.
- S. A. David, M. S. Smith, G. Lopez, S. Mukherjee, S. Buch and O. Narayan, AIDS Res. Hum. Retroviruses, 2001, 17, 59 CrossRef CAS PubMed.
- M. R. Kimbrell, H. Warshakoon, J. R. Cromer, S. Malladi, J. D. Hood, R. Balakrishna, T. A. Scholdberg and S. A. David, Immunol. Lett., 2008, 118, 132 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of intermediates and final compounds (1H, 13C, mass spectra). See DOI: 10.1039/c3ob40816g |
| This journal is © The Royal Society of Chemistry 2013 |

