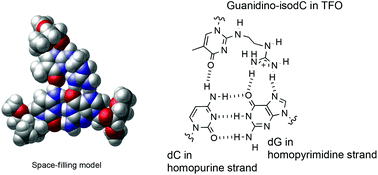
The development of novel nucleoside analogues for the formation of triplex DNA containing pyrimidine–purine inversion sites has been a challenging field. In this paper, we describe the design and synthesis of non-natural nucleoside analogues, N-substituted-2′-deoxy-5-methylisocytidine derivatives, and their evaluation for triplex formation. It has been shown that N-(guanidinoethyl)-2′-deoxy-5-methylisocytidine exhibits selective recognition of a CG interrupting site and potentiates the formation of anti-parallel triplexes.