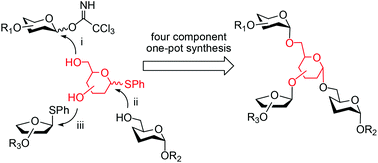
We describe in this paper the efficient four-component one-pot synthesis of three fully protected oligosaccharides 22, 36, and 50 with di-branched structures by employing D-galacto- and mannopyranosyl thioglycoside diols as central glycosylating agents. After global deprotection, they were converted respectively into the 3-aminopropyl linker-containing free oligosaccharide fragments 14, 24, and 38 structurally related to cell wall oligosaccharides from Atractylodes lancea DC, the marine fungus Lineolata rhizophorae and pathogenic Mycobacterium tuberculosis. The 3-aminopropyl linker at the anomeric carbon can enable conjugation of these synthetic oligomers to a suitable protein carrier.