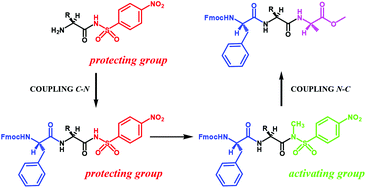
In this paper we describe a solution phase peptide synthesis strategy using the 4-nitrobenzenesulfonamido/N-methyl-4-nitrobenzenesulfonamido group as a protecting/activating system of the carboxyl function. The 4-nitrobenzenesulfonamido group is stable during peptide chain elongation (Fmoc chemistry). The N-aminoacyl or N-dipeptidyl-4-nitrobenzensulfonamides, when activated by methylation, can be easily coupled with another amino acid or reconverted into the free-carboxyl function amino acids or peptides. This activatable protecting group allows both the C → N and the N → C direction solution phase peptide synthesis. We also verified that the absolute configuration at the chiral centers does not change during the coupling reactions.