Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of imidazo and benzimidazo[2,1-a]isoquinolines by rhodium-catalyzed intramolecular double C–H bond activation†
Vutukuri Prakash
Reddy
,
Takanori
Iwasaki
and
Nobuaki
Kambe
*
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, Suita, Osaka 565-0871, Japan. E-mail: kambe@chem.eng.osaka-u.ac.jp; Fax: +81-6-6879-7390; Tel: +81-6-6879-7390
First published on 28th February 2013
Abstract
The rhodium-catalyzed intramolecular direct arylation of imidazole and benzimidazole derivatives via double C–H bond activation is described. This approach provides new access to a wide range of imidazo and benzimidazo[2,1-a]isoquinoline derivatives in moderate to high yields. This reaction provides an alternative method to the known Pd-catalyzed intramolecular oxidative cross-coupling reactions.
Introduction
Nitrogen-containing heterocycles and their derivatives are often found in natural products and in pharmaceuticals and agrochemicals.1,2 Since imidazo and benzimidazo[2,1-a]isoquinoline derivatives show interesting biological activities such as anticancer, anti-HIV-1, antimicrobial and antiviral properties,3,4 a variety of synthetic protocols have been developed for the preparation of imidazo and benzimidazo[2,1-a]isoquinoline derivatives based on condensation reactions5 and the photocyclization of 1-styrylimidazoles.6 In addition, coupling reactions catalyzed by transition metals, such as palladium and copper, have recently been reported and successfully applied to the preparation of such heterocyclic compounds through C–N and C–C cross-coupling.7 However, these protocols require the use of organohalides7a–d or boranes7e or both7f as starting materials. These prefunctionalized starting materials are not readily available from commercial sources and are often difficult to synthesize. Therefore, the development of more general and convenient processes using readily accessible and inexpensive substrates is an important theme. As a promising strategy for the synthesis of the imidazo and benzimidazo[2,1-a]isoquinoline derivatives, the intramolecular oxidative cross-coupling via cleavage of two C–H bonds would be a more straightforward and efficient route to these compounds.It has long been known that Pd catalyzes the oxidative homo-coupling of arenes8a,b and cross-coupling between arenes and olefins.8c,d This transformation has been successfully applied to cyclization by intramolecular oxidative cross-coupling reactions.8e–m This successful Pd-catalyzed functionalization of C–H bonds8n,o prompted the study of Rh catalyzed systems leading to the recent remarkable development of useful transformations via C–H bond cleavage9 such as oxidative Heck-type reactions,10 oxidative aryl–aryl coupling,11 as well as addition to carbon–carbon10c,d,k,q,12 or carbon–heteroatom13 unsaturated bonds.
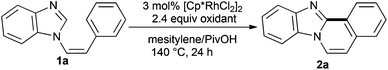
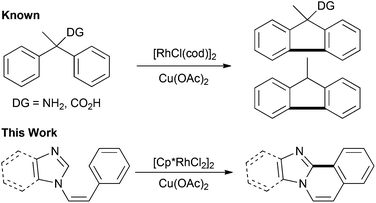
During the course of our study on transition metal-catalyzed cross-coupling reactions,14 we developed a straightforward procedure for the synthesis of imidazo and benzimidazo[2,1-a]isoquinoline derivatives. The method involves a rhodium-catalyzed cross-coupling reaction via double C–H bond cleavage as the first example of the rhodium-catalyzed intramolecular oxidative cross-coupling of heteroarenes with arenes, although the corresponding aryl–aryl intramolecular coupling reactions are known (Scheme 1).15
 | ||
| Scheme 1 Rhodium-catalyzed intramolecular oxidative cross-coupling reaction. | ||
Results and discussion
We carried out the reaction under different conditions using (Z)-1-styryl-1H-benzimidazole (1a) as a model substrate and the results are summarized in Table 1. Among the various solvents tested, DMA and mesitylene afforded the best results (Table 1, entries 1, 2, 5, 8), whereas DMF and toluene gave lower yields (entries 3, 4, 9, 10). PivOH was found to be an effective additive (entry 5). Among the Rh complexes examined, [Cp*RhCl2]2 proved to be preeminent for this reaction (entries 5, 12–16). When the amount of [Cp*RhCl2]2 was reduced to half from 3.0 mol%, the yield decreased (entries 5 and 17). No reaction took place in the absence of the metal catalyst (entry 18). The reaction was slow at 70 °C and the coupling product was produced in low yield (entry 19).|
|
||||
|---|---|---|---|---|
| Entry | Catalyst | Solvent | Additive | Yieldb (%) |
| a Reaction conditions: 1a (0.25 mmol), [Cp*RhCl2]2 (3.0 mol%), Cu(OAc)2 (0.6 mmol), additive (0.5 mmol), solvent (1.0 mL), 24 h, 140 °C. b Isolated yields. c Rh (10 mol%). d [Cp*RhCl2]2 (1.5 mol%). e At 70 °C. Cp*: pentamethylcyclopentadienyl; cod: 1,5-cyclooctadiene; nbd: bicyclo[2.2.1]hepta-2,5-diene. | ||||
| 1 | [Cp*RhCl2]2 | DMA | None | 49 |
| 2 | [Cp*RhCl2]2 | Mesitylene | None | 56 |
| 3 | [Cp*RhCl2]2 | DMF | None | 18 |
| 4 | [Cp*RhCl2]2 | Toluene | None | 25 |
| 5 | [Cp*RhCl2]2 | Mesitylene | PivOH | 77 |
| 6 | [Cp*RhCl2]2 | Mesitylene | AcOH | 65 |
| 7 | [Cp*RhCl2]2 | Mesitylene | 1-AdCO2H | 72 |
| 8 | [Cp*RhCl2]2 | DMA | PivOH | 63 |
| 9 | [Cp*RhCl2]2 | DMF | PivOH | 30 |
| 10 | [Cp*RhCl2]2 | Toluene | PivOH | 42 |
| 11 | [Cp*RhCl2]2 | None | PivOH | 26 |
| 12 | [Rh(cod)Cl]2 | Mesitylene | PivOH | 71 |
| 13c | [Rh(nbd)Cl]2 | Mesitylene | PivOH | 46 |
| 14c | Rh2(OAc)4 | Mesitylene | PivOH | 49 |
| 15c | Rh(PPh3)3Cl | Mesitylene | PivOH | 42 |
| 16c | RhCl3 | Mesitylene | PivOH | 36 |
| 17d | [Cp*RhCl2]2 | Mesitylene | PivOH | 48 |
| 18 | None | Mesitylene | PivOH | 0 |
| 19e | [Cp*RhCl2]2 | Mesitylene | PivOH | 31 |
Table 2 shows the effect of various oxidants on this reaction and Cu(OAc)2 was found to be an excellent oxidant (entry 6). The use of AgOAc gave products in low yield (entry 1) and other oxidants such as Ag2CO3, AgF, CuCl2, CuCO3, (NH4)2S2O8, TBHP (tert-butyl hydroperoxide) and K2S2O8 were ineffective (entries 2–5 and 10–12). In the absence of an oxidant, no reaction occurred (entry 13). The best yield was obtained when 3.0 mol% amount of [Cp*RhCl2]2 was employed in combination with Cu(OAc)2 (2.4 equiv.) as the oxidant and PivOH (0.5 mmol) as the additive in mesitylene (1.0 mL) at 140 °C for 24 h (entry 6). This reaction proceeded similarly in the absence of PivOH to give a comparative yield of product 2a (entry 7).
|
|
|||||
|---|---|---|---|---|---|
| Entry | Oxidant | Yieldb (%) | Entry | Oxidant | Yieldb (%) |
| a Reaction conditions: 1a (0.25 mmol), [Cp*RhCl2]2 (3.0 mol%), oxidants (0.6 mmol), PivOH (0.5 mmol), mesitylene (1.0 mL), 24 h, 140 °C. b Isolated yields. c [Cp*RhCl2]2 (5.0 mol%), Cu(OAc)2·H2O (0.3 mmol) and AgSbF6 (20 mol%) in mesitylene (1.0 mL) without using PivOH. d Cu(OAc)2 (0.3 mmol). e Cu(OAc)2 (0.45 mmol). f 2.0 equiv. | |||||
| 1 | AgOAc | 21 | 8d | Cu(OAc)2 | 47 |
| 2 | Ag2CO3 | Trace | 9e | Cu(OAc)2 | 65 |
| 3 | AgF | 0 | 10 | (NH4)2S2O8 | 0 |
| 4 | CuCl2 | Trace | 11f | TBHP | 0 |
| 5 | CuCO3 | Trace | 12 | K2S2O8 | 0 |
| 6 | Cu(OAc)2 | 77 | 13 | — | 0 |
| 7 | Cu(OAc)2·H2O | 81 | |||
To explore the scope of this rhodium-catalyzed intramolecular oxidative cross-coupling, the reactions of 1a–n were examined under optimized conditions. As shown in Table 3, the corresponding imidazo and benzimidazo[2,1-a]isoquinoline derivatives 2a–l were obtained in moderate to high yields. Alkyl and methoxy substituents exerted little effect on the yield, but substituents at the ortho position of the benzene ring slightly reduced the yields due to the steric effect (2c and 2j). When 1h carrying a 1,2-diphenylvinyl moiety was employed, the reaction proceeded efficiently to give the corresponding cyclized product 2h in 62% yield, indicating that the phenyl group on the vinylic tether carbon did not affect the reaction.16 However, 1m, having a thiophene ring, and 1n, having an (E)-styryl group, did not afford coupling products. The procedure using AgSbF6 and Cu(OAc)2·H2O in mesitylene gave slightly better results than the procedure using PivOH as the additive as shown in Table 3.
A plausible catalytic cycle for the reaction is illustrated in Scheme 2. The reaction starts from Cp*Rh(OPiv)2 or Cp*Rh (OAc)2 which is derived from the ligand exchange between rhodium dimer and the pivalic acid or Cu(OAc)2. Thus formed Cp*Rh(OCOR)2 is coordinated with a nitrogen lone pair of imidazole to give intermediate II in Path A. Rh then undergoes insertion into the C–H bond at the C2 position assisted by the carboxylate ligand leading to the formation of intermediate III with the loss of carboxylic acid.17 The subsequent C–H bond cleavage by Rh(III) generates a seven-membered rhodacyclic intermediate IV which undergoes reductive elimination to give product 2 and a Rh(I) species, and the latter is oxidized by Cu(II) to complete the catalytic cycle. A possible alternative pathway is shown by Path B, where the catalytic cycle is triggered by the coordination of the nitrogen lone pair of imidazole to Cu(OCOR)2 affording intermediate V. The insertion of Cu into a C–H bond followed by transmetalation with rhodium then gives intermediate IIIviaVI.18
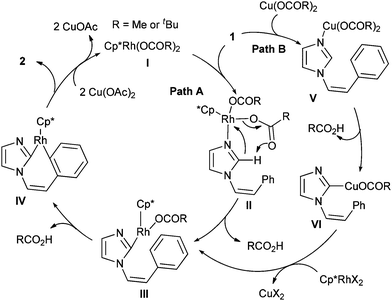 | ||
| Scheme 2 Plausible reaction pathways of rhodium-catalyzed oxidative cross-coupling. | ||
Conclusions
We report herein on the rhodium-catalyzed synthesis of imidazo and benzimidazo[2,1-a]isoquinolines via the intramolecular oxidative cross-coupling reaction through double C–H bond cleavage. This protocol can be applied to the synthesis of various heterocyclic compounds. The scope of the reaction and further applications as well as mechanistic studies of the rhodium-catalyzed C–H activation reactions are currently under investigation.Acknowledgements
This work was supported by a GCOE and Grant-in-Aid for Scientific Research (S) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.Notes and references
- Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky and C. W. Rees, Elsevier, New York, 1984, vol. 4, 1–38 Search PubMed.
- Z. Jin, Nat. Prod. Rep., 2005, 22, 196 RSC.
- (a) R. L. Weinkauf, A. Y. Chen, C. Yu, L. Liu, L. Barrows and E. J. Lavoie, Bioorg. Med. Chem., 1994, 2, 781 CrossRef CAS; (b) W. J. Houlihan, P. G. Munder, D. A. Handley, S. H. Cheon and V. A. Parrino, J. Med. Chem., 1995, 38, 234 CrossRef CAS; (c) P. Molina, E. Aller, A. Lorenzo, C. Foces and A. L. L. Saiz, Tetrahedron, 1996, 52, 13671 CrossRef CAS; (d) Z. X. Lu, N. H. Quazi, L. W. Deady and G. M. Polya, Biol. Chem. Hoppe-Seyler, 1996, 377, 373 CrossRef CAS; (e) V. K. Pandey and A. Shukla, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1999, 38, 1381 Search PubMed; (f) L. W. Deady, T. Rodemann, G. J. Finlay, B. C. Baguley and W. A. Denny, Anti-Cancer Drug Des., 2000, 15, 339 CAS; (g) S. M. Rida, S. A. M. El-Hawash, H. T. Y. Fahmy, A. A. Hazzaa and M. M. M. El-Meligy, Arch. Pharmacal Res., 2006, 29, 826 CrossRef CAS.
- I. Hayakawa, Y. Sugano, T. Agatsuma, H. Furukawa, S. Kurakata and S. Naruto, PCT Int. Appl., WO2002034748, 2002 Search PubMed; Chem. Abstr. 2002 136 355236 Search PubMed.
- (a) A. R. Katritzky, G. Qiu, Q.-H. Long, H.-Y. He and P. J. Steel, J. Org. Chem., 2000, 65, 9201 CrossRef CAS; (b) G. Dyker, W. Stirner and G. Henkel, Eur. J. Org. Chem., 2000, 1433 CrossRef CAS; (c) L. W. Deady and S. M. Devine, J. Heterocycl. Chem., 2004, 41, 549 CrossRef CAS; (d) R.-S. Hou, H.-M. Wang, H.-Y. Huang and L.-C. Chen, J. Chin. Chem. Soc., 2004, 51, 1417 CAS; (e) A. D. C. Parenty, Y.-F. Song, C.-J. Richmond and L. Cronin, Org. Lett., 2007, 9, 2253 CrossRef CAS; (f) N. Okamoto, K. Sakurai, M. Ishikura, K. Takeda and R. Yanada, Tetrahedron Lett., 2009, 50, 4167 CrossRef CAS; (g) N. Chernyak and V. Gevorgyan, Angew. Chem., Int. Ed., 2010, 49, 2743 CrossRef CAS.
- G. Cooper and W. J. Irwin, J. Chem. Soc., Perkin Trans. 1, 1976, 75 RSC.
- (a) Q. Cai, Z. Li, J. Wei, L. Fu, C. Ha, D. Pei and K. Ding, Org. Lett., 2010, 12, 1500 CrossRef CAS; (b) S. Nandi, S. Samanta, S. Jana and J. K. Ray, Tetrahedron Lett., 2010, 51, 5294 CrossRef CAS; (c) B. Liu, X.-R. Qin, K.-Z. Li, X.-Y. Li, Q. Guo, J.-B. Lan and J.-S. You, Chem.–Eur. J., 2010, 16, 11836 CrossRef CAS; (d) I. Cerna, R. Pohl, B. Klepetarova and M. Hocek, J. Org. Chem., 2010, 75, 2302 CrossRef CAS; (e) J. Lu and H. Fu, J. Org. Chem., 2011, 76, 4600 CrossRef CAS; (f) Q. Liao, L.-Y. Zhang, S.-T. Li and C.-J. Xi, Org. Lett., 2011, 13, 228 CrossRef CAS.
- For selected reports on palladium-catalyzed C–H activation reactions see: (a) A. Shiotani and H. Itatani, Angew. Chem., Int. Ed. Engl., 1974, 13, 471 CrossRef; (b) B. Akermark, L. Eberson, E. Jonsson and E. Petterson, J. Org. Chem., 1975, 40, 1365 CrossRef CAS; (c) Y. Fujiwara, I. Moritani, S. Danno, R. Asano and S. Teranishi, J. Am. Chem. Soc., 1969, 91, 7166 CrossRef CAS; (d) H. Iida, Y. Yuasa and C. Kibayashi, J. Org. Chem., 1980, 45, 2938 CrossRef CAS; (e) T. Watanabe, S. Ueda, S. Inuki, S. Oishi, N. Fujii and H. Ohno, Chem. Commun., 2007, 4516 RSC; (f) B. Liegault, D. Lee, M. P. Huestis, D. R. Stuart and K. Fagnou, J. Org. Chem., 2008, 73, 5022 CrossRef CAS; (g) S. Wurtz, S. Rakshit, J. J. Neumann, T. Droge and F. Glorius, Angew. Chem., Int. Ed., 2008, 47, 7230 CrossRef; (h) H.-J. Knolker, Chem. Lett., 2009, 38, 8 CrossRef; (i) L. Ackermann, R. Jeyachandran, H. K. Potukuchi, P. Novak and L. Buttner, Org. Lett., 2010, 12, 2056 CrossRef CAS; (j) D. G. Pintori and M. F. Greaney, J. Am. Chem. Soc., 2011, 133, 1209 CrossRef CAS; (k) P. Gandeepan, C.-H. Hung and C.-H. Cheng, Chem. Commun., 2012, 48, 9379 RSC; (l) G. Meng, H.-Y. Niu, G.-R. Qu, J. S. Fossey, J.-P. Li and H.-M. Guo, Chem. Commun., 2012, 48, 9601 RSC; (m) M. Sun, H. Wu, J. Zheng and W. Bao, Adv. Synth. Catal., 2012, 354, 835 CrossRef CAS . For recent reviews, see: ; (n) S.-L. You and J.-B. Xia, Top. Curr. Chem., 2010, 292, 165 CrossRef CAS; (o) N. Kuhl, M. N. Hopkinson, J. Wencel-Delord and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 10236 CrossRef CAS.
- For recent reviews on rhodium(III)-catalyzed C–H activation reactions, see: (a) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef CAS; (b) J. Bouffard and K. Itami, Top. Curr. Chem., 2010, 292, 231 CrossRef CAS; (c) T. Satoh and M. Miura, Chem.–Eur. J., 2010, 16, 11212 CrossRef CAS; (d) T. Satoh and M. Miura, Synthesis, 2010, 3395 CrossRef CAS; (e) D. A. Colby, A.-S. Tsai, R. G. Bergman and J. A. Ellman, Acc. Chem. Res., 2012, 45, 814 CrossRef CAS.
- (a) T. Matsumoto and H. Toshida, Chem. Lett., 2000, 1064 CrossRef CAS; (b) T. Matsumoto, R. A. Periana, D. Taube and H. Yoshida, J. Catal., 2002, 206, 272 CrossRef CAS; (c) K. Ueura, T. Satoh and M. Miura, Org. Lett., 2007, 9, 1407 CrossRef CAS; (d) J. Chen, G. Song, C.-L. Pan and X. Li, Org. Lett., 2010, 12, 5426 CrossRef CAS; (e) F. Wang, G. Song and X. Li, Org. Lett., 2010, 12, 5430 CrossRef CAS; (f) A. S. Tsai, M. Brasse, R. G. Bergman and J. A. Ellman, Org. Lett., 2011, 13, 540 CrossRef CAS; (g) F. W. Patureau, T. Besset and F. Glorius, Angew. Chem., Int. Ed., 2011, 50, 1064 CrossRef CAS; (h) S. Rakshit, C. Grohmann, T. Besset and F. Glorius, J. Am. Chem. Soc., 2011, 133, 2350 CrossRef CAS; (i) H. Li, Y. Li, X.-S. Zhang, K. Chen, X. Wang and Z.-J. Shi, J. Am. Chem. Soc., 2011, 133, 15244 CrossRef CAS; (j) S. Mochida, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2011, 76, 3024 CrossRef CAS; (k) X. Li and M. Zhao, J. Org. Chem., 2011, 76, 8530 CrossRef CAS; (l) S. H. Park, J. Y. Kim and S. Chang, Org. Lett., 2011, 13, 2372 CrossRef CAS; (m) F. Wang, G. Song, Z. Du and X. Li, J. Org. Chem., 2011, 76, 2926 CrossRef CAS; (n) J. Willwacher, S. Rakshit and F. Glorius, Org. Biomol. Chem., 2011, 9, 4736 RSC; (o) S. Mochida, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2011, 76, 3024 CrossRef CAS; (p) L. Zheng and J. Wang, Chem.–Eur. J., 2012, 18, 9699 CrossRef CAS; (q) P. Zhao, R. Niu, F. Wang and X. Li, Org. Lett., 2012, 14, 4166 CrossRef CAS; (r) Z. Shi, N. Schrçder and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 8092 CrossRef CAS.
- (a) K. Morimoto, M. Itoh, K. Hirano, T. Satoh, Y. Shibata, K. Tanaka and M. Miura, Angew. Chem., Int. Ed., 2012, 51, 5359 CrossRef CAS; (b) J. Wencel-Delord, C. Nimphius, F. W. Patureau and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 2247 CrossRef CAS; (c) P. Wang, H. Rao, R. Hua and C.-J. Li, Org. Lett., 2012, 14, 902 CrossRef CAS; (d) N. Kuhl, M. N. Hopkinson and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 8230 CrossRef CAS; (e) J. Dong, Z. Long, F. Song, N. Wu, Q. Guo, J. Lan and J. You, Angew. Chem., Int. Ed., 2013, 52, 580 CrossRef CAS; (f) V. P. Reddy, R. Qiu, T. Iwasaki and N. Kambe, Org. Lett. DOI:10.1021/ol400230y.
- (a) K. Ueura, T. Satoh and M. Miura, J. Org. Chem., 2007, 72, 5362 CrossRef CAS; (b) N. Umeda, H. Tsurugi, T. Satoh and M. Miura, Angew. Chem., Int. Ed., 2008, 47, 4019 CrossRef CAS; (c) T. Fukutani, N. Umeda, K. Hirano, T. Satoh and M. Miura, Chem. Commun., 2009, 5141 RSC; (d) M. Shimizu, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2009, 74, 3478 CrossRef CAS; (e) T. K. Hyster and T. Rovis, J. Am. Chem. Soc., 2010, 132, 10565 CrossRef CAS; (f) P. C. Too, Y.-F. Wang and S. Chiba, Org. Lett., 2010, 12, 5688 CrossRef CAS; (g) K. Morimoto, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2010, 12, 2068 CrossRef CAS; (h) N. Guimond, S. I. Gorelsky and K. Fagnou, J. Am. Chem. Soc., 2011, 133, 6449 CrossRef CAS; (i) T. Fukutani, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2011, 76, 2867 CrossRef CAS; (j) N. Umeda, K. Hirano, T. Satoh, N. Shibata, H. Sato and M. Miura, J. Org. Chem., 2011, 76, 13 CrossRef CAS; (k) F. W. Patureau, T. Besset, N. Kuhl and F. Glorius, J. Am. Chem. Soc., 2011, 133, 2154 CrossRef CAS; (l) K. Morimoto, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2011, 76, 9548 CrossRef CAS; (m) H. Wang and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 7318 CrossRef CAS.
- (a) K. D. Hesp, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2011, 133, 11430 CrossRef CAS; (b) Y. Li, B.-J. Li, W.-H. Wang, W.-P. Huang, X.-S. Zhang, K. Chen and Z.-J. Shi, Angew. Chem., Int. Ed., 2011, 50, 2115 CrossRef CAS; (c) L. Yang, C. A. Correia and C.-J. Li, Adv. Synth. Catal., 2011, 353, 1269 CrossRef CAS.
- (a) J. Terao, A. Oda and N. Kambe, Org. Lett., 2004, 6, 3341 CrossRef CAS; (b) Y. Minami, H. Kuniyasu and N. Kambe, Org. Lett., 2008, 10, 2469 CrossRef CAS; (c) M. Toyofuku, S.-I. Fujiwara, T. Shin-ike, H. Kuniyasu and N. Kambe, J. Am. Chem. Soc., 2005, 127, 9706 CrossRef CAS; (d) M. Toyofuku, E. Murase, S.-I. Fujiwara, T. Shin-ike, H. Kuniyasu and N. Kambe, Org. Lett., 2008, 10, 3957 CrossRef CAS; (e) Y. Minami, H. Kuniyasu, A. Sanagawa and N. Kambe, Org. Lett., 2010, 12, 3744 CrossRef CAS; (f) N. Kambe, T. Iwasaki and J. Terao, Chem. Soc. Rev., 2011, 40, 4937 RSC.
- Recently, palladium-catalyzed intermolecular oxidative cross-coupling of imidazole and benzimidazole derivatives has been reported: ref. 8m. For Rh-catalyzed intramolecular aryl–aryl coupling, see: ref. 11a,c.
- In ref. 8m, it was claimed that introduction of a Me substituent on an olefinic tether carbon resulted in lower yield (21%) in the case of the Pd/Cu catalytic system.
- D. Lapointe and K. Fagnou, Chem. Lett., 2010, 39, 1118 CrossRef.
- J.-R. Huang, L. Dong, B. Han, C. Peng and Y.-C. Chen, Chem.–Eur. J., 2012, 18, 8896 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details, characterization data, 1H and 13C NMR spectra for new products. See DOI: 10.1039/c3ob27396b |
| This journal is © The Royal Society of Chemistry 2013 |