Phyllis A. Leber, Ryan M. Bell, Carlton W. Christie and Joseph A. Mohrbacher III
Org. Biomol. Chem., 2013,11, 2080-2083
DOI:
10.1039/C3OB00033H,
Communication
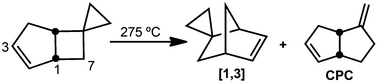
Appending a spirocyclopropane linkage to