N-heterocyclic carbene-catalyzed [4 + 2] cycloaddition of ketenes and 3-aroylcoumarins: highly enantioselective synthesis of dihydrocoumarin-fused dihydropyranones†
Teng-Yue
Jian
,
Xiang-Yu
Chen
,
Li-Hui
Sun
and
Song
Ye
*
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: songye@iccas.ac.cn; Fax: (+86) 10 6255 4449; Tel: (+86) 10 6264 1156
First published on 16th October 2012
Abstract
The N-heterocyclic carbene-catalyzed [4 + 2] cyclization of ketenes and 3-aroylcoumarins has been developed to give the corresponding dihydrocoumarin-fused multisubstituted dihydropyranones in high yield with good diastereoselectivity and high enantioselectivity.
Introduction
Compounds with two or more heterocycles fused play a vital role in natural and unnatural bioactive compounds.1 Coumarins are present in large quantities in human diets and, more importantly, they are the active molecules in many traditional herbal medicines.2 Thus the synthesis of coumarin and its derivatives attracts intensive attention.3 In this paper, we wish to report an N-heterocyclic carbene-catalyzed cycloaddition reaction of ketenes with aroylcoumarins for the construction of dihydrocoumarin-fused dihydropyranones.Since Staudinger's discovery of ketenes in the early 1900s,4 their cycloaddition reactions have become a powerful methodology for the construction of cyclic compounds.5,6 Over the past decades, N-heterocyclic carbene (NHC) catalysis has been very successful for a variety of reactions.7 We,8 independently with Smith et al.,9 demonstrated that N-heterocyclic carbenes (NHCs) were efficient catalysts for the formal cycloaddition reactions of ketenes. In 2008 and later, we reported the NHC-catalyzed enantioselective [4 + 2] cycloaddition of ketenes with activated enones, giving the corresponding dihydropyranones in good yield with high enantioselectivity.10 We envisaged that 3-aroylcoumarin may act as the oxodiene for the NHC-catalyzed [4 + 2] cycloaddition of ketenes, and thus provide a facile enantioselective access to coumarin-fused dihydropyranones.
Results and discussion
Initially, the reaction of phenyl(ethyl)ketene (1a) and 3-benzoyl-2H-chromen-2-one (2a) was investigated under NHC catalysis (Table 1). We were encouraged to find that the desired [4 + 2] cycloadduct 3aa was isolated in 49% yield with 29% ee and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr in the presence of 10 mol% NHC 4a′,8a,11 generated from L-pyroglutamic acid derived triazolium salt 4a and 20 mol% of Cs2CO3 (entry 1). NHC 4b′ with a less bulky TMS group and NHC 4c′ with a bulky N-(2-isopropyl)phenyl group resulted in better yields but decreased enantioselectivity (entries 2 and 3). NHC 4d′ with a free hydroxyl group12 led to a reversed but high enantioselectivity (80% ee) for this reaction (entry 4). Tetracyclic NHC 5′, derived from aminoindanol, also worked well in the reaction, giving the cycloadduct 3aa in 88% yield with 67% ee and 8
1 dr in the presence of 10 mol% NHC 4a′,8a,11 generated from L-pyroglutamic acid derived triazolium salt 4a and 20 mol% of Cs2CO3 (entry 1). NHC 4b′ with a less bulky TMS group and NHC 4c′ with a bulky N-(2-isopropyl)phenyl group resulted in better yields but decreased enantioselectivity (entries 2 and 3). NHC 4d′ with a free hydroxyl group12 led to a reversed but high enantioselectivity (80% ee) for this reaction (entry 4). Tetracyclic NHC 5′, derived from aminoindanol, also worked well in the reaction, giving the cycloadduct 3aa in 88% yield with 67% ee and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (entry 5).
1 dr (entry 5).
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Entry | 4–5 (X)a | Cs2CO3 (Y) | Solvent | T (°C) | Yieldb (%) | eec (%) | dre |
| a NHC 4′–5′ was generated from its precursor 4–5 (10–12 mol%) in the presence of Cs2CO3 (10–20 mol%) in the noted solvent at room temperature for 30 min. b Isolated yield of pure cis-3aa. c Determined by HPLC. d ent-3aa was isolated as the major enantiomer for entries 1–3. e Determined by 1H NMR (300 MHz) of the reaction mixture. | |||||||
| 1 | 4a (10) | 20 | DCM | rt | 49 | 29d | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 4b (10) | 20 | DCM | rt | 77 | 6d | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 4c (10) | 20 | DCM | rt | 82 | 18d | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 4d (10) | 20 | DCM | rt | 81 | 80 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 5 (10) | 20 | DCM | rt | 88 | 67 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 4d (10) | 20 | THF | rt | 67 | 10 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 4d (10) | 20 | Toluene | rt | 89 | 87 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 4d (10) | 20 | Toluene | 0 °C | 91 | 83 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 4d (12) | 10 | Toluene | 0 °C | 87 | 91 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Solvent screening revealed that the reaction performed better in toluene than in DCM or THF in terms of enantioselectivity (87% ee vs. 80% ee or 10% ee, entries 4, 6–7). Lowering the reaction temperature to 0 °C benefits the yield but with decreased enantioselectivity (entry 8). Careful examination revealed that excess Cs2CO3 could also promote the reaction, giving the cycloadduct as a racemate. Thus the reaction employing 10 mol% of Cs2CO3 and 12 mol% triazolium NHC precursor 4d resulted in an improved enantioselectivity (91% ee) and diastereoselectivity (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) (entry 9).
1 dr) (entry 9).
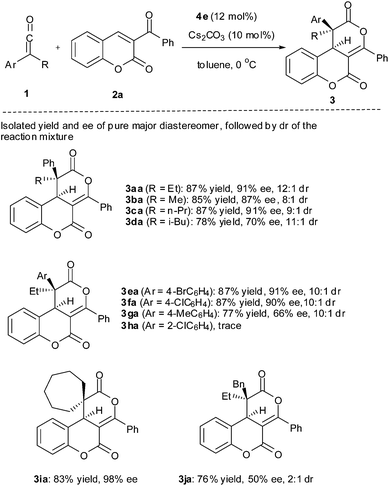
With the optimized reaction condition in hand, the reaction scope was then briefly investigated (Table 2). It was found that aryl(alkyl)ketenes with ethyl, methyl and n-propyl all worked well to give the corresponding cycloadduct (3aa, 3ba and 3ca) in high yield with high enantioselectivity and diastereoselectivity. However, ketenes with a bulky isobutyl group resulted in decreased yield and enantioselectivity (3da). Electron-withdrawing substituents (4-Br, 4-ClC6H4) are well tolerated, giving cycloadducts (3ea and 3fa) in high yield with high enantioselectivity, while an electron-donating group (4-MeC6H4) led to some loss of yield and enantioselectivity (3ga). It should be noted that reaction of ketene containing an o-chlorophenyl group gave only a trace of cycloaddduct (3ha). Interestingly, ketene 1i derived from cycloheptanecarbonyl chloride worked very well, giving the desired cycloadduct 3ia in 83% yield with 98% ee, while benzyl(ethyl)ketene (1j) resulted in decreased yield and low diastereo- and enantioselectivity (3ja).
|
Several 3-aroylcoumarins were also tested for the reaction (Table 3). Both an electron-donating group (4-MeC6H4) and electron-withdrawing groups (4-BrC6H4, 4-ClC6H4) on the aroyl are tolerated, giving the desired cycloadduct in high yield with good diastereo- and enantioselectivity (3ab, 3ac and 3ad). However, aroyl groups with meta- or ortho-substituents (3-ClC6H4, 2-ClC6H4) led to decreased enantioselectivity albeit in high yield and good to high diastereoselectivity (3ae and 3af). 6-Halocoumarin derivatives (X = Cl, Br) also worked as well as the parent one (3ag and 3ah).
The absolute stereochemistry of cycloadduct (+)-3fa was unambiguous established by the X-ray analysis† of its crystal (Fig. 1),13 and that of all other cycloadducts is proposed by analogy. The crystal of (+)-3fa was prepared from a solution in DCM–ether (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) with a trace of petroleum ether.
10) with a trace of petroleum ether.
 | ||
| Fig. 1 X-ray structure of cycloadduct (+)-3fa. | ||
Based on the dramatic effect of the free hydroxyl group of the NHC catalyst, and the diastereo- and enantioselectivity observed,14 a likely transition state is proposed as in Fig. 2. The enolate generated by addition of the NHC to ketene favors its Z-isomer, which minimizes the steric repulsion. The hydrogen-bonding between the hydroxy group of the NHC–ketene adduct and aroyl group of the coumarin derivative directs the facial selectivity. The endo transition state of the Diels–Alder reaction is favored and results in the cis-cycloadduct observed.
 | ||
| Fig. 2 Proposed model for stereochemical outcome. | ||
Conclusions
In summary, N-heterocyclic carbenes were found to be efficient catalysts for the [4 + 2] cycloaddition of ketenes and 3-aroylcoumarins. When the NHC derived from L-pyroglutamic acid and featuring a free hydroxyl group was used as the catalyst, the desired dihydrocoumarin-fused dihydropyranones were obtained in high yield with good to high diastereo- and enantioselectivity.Experimental
Typical procedure for NHC-catalyzed [4 + 2] cycloaddition of ketenes with 3-aroylcoumarins
An oven-dried 50 mL Schlenk tube equipped with a stir bar was charged with triazolium salt 4d (27.3 mg, 0.06 mmol) and anhydrous Cs2CO3 (17 mg, 0.05 mmol). This tube was closed with a septum, evacuated, and back-filled with argon. To this mixture was added freshly distilled toluene (5 mL) and stirred for 30 min at room temperature. After stirring for 10 min at 0 °C, ketene 1a (146 mg, 1 mmol) and 3-aroylcoumarin 2a (125 mg, 0.5 mmol) were added. After stirring for 24 h, the reaction mixture was diluted with diethyl ether and passed through a short silica pad. The solvent was removed under reduced pressure and the residue was purified by chromatography on silica gel (ethyl acetate–petroleum ether, typically 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) to give the desired product.
100) to give the desired product.
Racemic samples for the standard of chiral HPLC spectra were prepared using 2-phenyl-6,7-dihydro-5H-pyrrolo[2,1-c][1,2,4]triazolium chloride as the catalyst.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +158.4 (c 1.2, CHCl3), HPLC analysis: 91% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90
1); [α]25D +158.4 (c 1.2, CHCl3), HPLC analysis: 91% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1.0 mL min−1, 254 nm, 16.3 min (major), 25.9 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.48–7.42 (m, 3H), 7.40–7.37 (m, 4H), 7.25–7.24 (m, 1H), 7.19–7.02 (m, 3H), 7.04 (d, J = 8.1 Hz, 1H), 6.73 (dd, J = 6.6 Hz, J = 1.8 Hz, 2H), 4.67 (s, 1H), 2.40–2.32 (m, 2H), 1.20 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 170.0, 164.0, 158.1, 151.7, 136.5, 131.6, 131.4, 130.0, 129.9, 129.4, 128.9, 128.3, 127.9, 126.0, 124.1, 117.1, 116.8, 104.1, 56.5, 40.2, 29.6, 10.2. IR (KBr) ν 3044, 1951, 1774, 1612, 1321, 785, 515, 468. HRMS (EI) m/z: M+ Calc. for C26H20O4, 396.1362, Found 396.1366.
10, 1.0 mL min−1, 254 nm, 16.3 min (major), 25.9 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.48–7.42 (m, 3H), 7.40–7.37 (m, 4H), 7.25–7.24 (m, 1H), 7.19–7.02 (m, 3H), 7.04 (d, J = 8.1 Hz, 1H), 6.73 (dd, J = 6.6 Hz, J = 1.8 Hz, 2H), 4.67 (s, 1H), 2.40–2.32 (m, 2H), 1.20 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 170.0, 164.0, 158.1, 151.7, 136.5, 131.6, 131.4, 130.0, 129.9, 129.4, 128.9, 128.3, 127.9, 126.0, 124.1, 117.1, 116.8, 104.1, 56.5, 40.2, 29.6, 10.2. IR (KBr) ν 3044, 1951, 1774, 1612, 1321, 785, 515, 468. HRMS (EI) m/z: M+ Calc. for C26H20O4, 396.1362, Found 396.1366.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +167.3 (c 1.0, CHCl3), HPLC analysis: 87% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90
1); [α]25D +167.3 (c 1.0, CHCl3), HPLC analysis: 87% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1.0 mL min−1, 254 nm, 12.3 min (major), 19.4 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.49–7.45 (m, 1H), 7.42–7.27 (m, 6H), 7.25–7.21 (m, 1H), 7.20–7.17 (m, 3H), 7.0–7.06 (m, 1H), 6.81–6.77 (m, 2H), 4.53 (s, 1H), 1.94 (s, 3H). 13C NMR (300 MHz, CDCl3) δ 170.8, 164.2, 157.9, 151.5, 137.4, 131.5, 131.3, 130.1, 129.8, 129.4, 129.0, 128.3, 127.9, 125.5, 124.1, 117.7, 116.7, 104.5, 52.0, 42.6, 25.0. IR (KBr) ν 3024, 1855, 1774, 1642, 1221, 775, 550, 468. HRMS (EI) m/z: M+ Calc. for C25H18O4, 382.1205, Found 382.1212.
10, 1.0 mL min−1, 254 nm, 12.3 min (major), 19.4 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.49–7.45 (m, 1H), 7.42–7.27 (m, 6H), 7.25–7.21 (m, 1H), 7.20–7.17 (m, 3H), 7.0–7.06 (m, 1H), 6.81–6.77 (m, 2H), 4.53 (s, 1H), 1.94 (s, 3H). 13C NMR (300 MHz, CDCl3) δ 170.8, 164.2, 157.9, 151.5, 137.4, 131.5, 131.3, 130.1, 129.8, 129.4, 129.0, 128.3, 127.9, 125.5, 124.1, 117.7, 116.7, 104.5, 52.0, 42.6, 25.0. IR (KBr) ν 3024, 1855, 1774, 1642, 1221, 775, 550, 468. HRMS (EI) m/z: M+ Calc. for C25H18O4, 382.1205, Found 382.1212.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +241 (c 1.5, CHCl3), HPLC analysis: 91% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 85
1); [α]25D +241 (c 1.5, CHCl3), HPLC analysis: 91% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 1.0 mL min−1, 254 nm, 11.5 min (major), 15.9 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.54–7.40 (m, 7H), 7.38–7.15 (m, 4H), 7.05 (d, J = 8.1 Hz, 1H), 6.74 (d, J = 6.0 Hz, 2H), 4.66 (s, 1H), 2.35 (t, J = 8.7 Hz, 2H), 1.96–1.86 (m, 1H), 1.36–1.23 (m, 1H), 1.02 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 170.1, 164.0, 158.1, 151.6, 136.6, 131.6, 131.4, 130.0, 129.8, 129.3, 128.8, 128.2, 127.9, 125.9, 124.1, 117.7, 116.8, 104.1, 56.1, 40.5, 38.9, 18.7, 14.4. IR (KBr) ν 3030, 1961, 1834, 1644, 1331, 765, 545, 455. HRMS (EI) m/z: M+ Calc. for C27H22O4, 410.1518, Found 410.1523.
15, 1.0 mL min−1, 254 nm, 11.5 min (major), 15.9 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.54–7.40 (m, 7H), 7.38–7.15 (m, 4H), 7.05 (d, J = 8.1 Hz, 1H), 6.74 (d, J = 6.0 Hz, 2H), 4.66 (s, 1H), 2.35 (t, J = 8.7 Hz, 2H), 1.96–1.86 (m, 1H), 1.36–1.23 (m, 1H), 1.02 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 170.1, 164.0, 158.1, 151.6, 136.6, 131.6, 131.4, 130.0, 129.8, 129.3, 128.8, 128.2, 127.9, 125.9, 124.1, 117.7, 116.8, 104.1, 56.1, 40.5, 38.9, 18.7, 14.4. IR (KBr) ν 3030, 1961, 1834, 1644, 1331, 765, 545, 455. HRMS (EI) m/z: M+ Calc. for C27H22O4, 410.1518, Found 410.1523.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +241.3 (c 1.0, CHCl3), HPLC analysis: 70% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 85
1); [α]25D +241.3 (c 1.0, CHCl3), HPLC analysis: 70% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 1.0 mL min−1, 254 nm, 10.2 min (major), 15.5 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.47–7.35 (m, 7H), 7.26–7.20 (m, 4H), 7.10 (d, J = 8.7 Hz, 1H), 6.94–6.91 (m, 2H), 4.58 (s, 1H), 2.30–2.17 (m, 2H), 2.04–1.95 (m, 1H), 1.09 (d, J = 7.5 Hz, 3H), 0.33 (d, J = 6.6 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 170.4, 164.0, 158.5, 151.5, 136.1, 131.5, 131.3, 130.1, 130.0, 129.7, 128.9, 128.4, 127.9, 126.6, 124.0, 117.7, 117.2, 104.1, 54.8, 45.7, 42.3, 25.9, 25.5, 23.6. IR (KBr) ν 3030, 1871, 1674, 1512, 1221, 780, 535. HRMS (EI) m/z: M+ Calc. for C28H24O4, 424.1675, Found 424.1682.
15, 1.0 mL min−1, 254 nm, 10.2 min (major), 15.5 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.47–7.35 (m, 7H), 7.26–7.20 (m, 4H), 7.10 (d, J = 8.7 Hz, 1H), 6.94–6.91 (m, 2H), 4.58 (s, 1H), 2.30–2.17 (m, 2H), 2.04–1.95 (m, 1H), 1.09 (d, J = 7.5 Hz, 3H), 0.33 (d, J = 6.6 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 170.4, 164.0, 158.5, 151.5, 136.1, 131.5, 131.3, 130.1, 130.0, 129.7, 128.9, 128.4, 127.9, 126.6, 124.0, 117.7, 117.2, 104.1, 54.8, 45.7, 42.3, 25.9, 25.5, 23.6. IR (KBr) ν 3030, 1871, 1674, 1512, 1221, 780, 535. HRMS (EI) m/z: M+ Calc. for C28H24O4, 424.1675, Found 424.1682.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +231.4 (c 1.5, CHCl3), HPLC analysis: 91% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90
1); [α]25D +231.4 (c 1.5, CHCl3), HPLC analysis: 91% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1.0 mL min−1, 254 nm, 15.6 min (major), 18.9 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.55–7.38 (m, 7H), 7.32–7.20 (m, 3H), 7.05 (d, J = 7.8 Hz, 1H), 6.61 (d, J = 5.7 Hz, 2H), 4.67 (s, 1H), 2.37–2.29 (m, 2H), 1.19 (t, J = 6.9 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.5, 164.0, 158.0, 151.65, 135.6, 132.0, 131.6, 131.3, 130.2, 129.9, 129.3, 128.0, 127.7, 124.3, 122.5, 117.8, 116.4, 103.9, 56.2, 39.9, 29.6, 10.1. IR (KBr) ν 2980, 1836, 1658, 1556, 1262, 755. HRMS (EI) m/z: M+ Calc. for C26H19Br79O4, 474.0467, Found 474.0475, M+ Calc. for C26H19Br81O4, 476.0446, Found 474.0443.
10, 1.0 mL min−1, 254 nm, 15.6 min (major), 18.9 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.55–7.38 (m, 7H), 7.32–7.20 (m, 3H), 7.05 (d, J = 7.8 Hz, 1H), 6.61 (d, J = 5.7 Hz, 2H), 4.67 (s, 1H), 2.37–2.29 (m, 2H), 1.19 (t, J = 6.9 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.5, 164.0, 158.0, 151.65, 135.6, 132.0, 131.6, 131.3, 130.2, 129.9, 129.3, 128.0, 127.7, 124.3, 122.5, 117.8, 116.4, 103.9, 56.2, 39.9, 29.6, 10.1. IR (KBr) ν 2980, 1836, 1658, 1556, 1262, 755. HRMS (EI) m/z: M+ Calc. for C26H19Br79O4, 474.0467, Found 474.0475, M+ Calc. for C26H19Br81O4, 476.0446, Found 474.0443.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +211.3 (c 1.4, CHCl3), HPLC analysis: 90% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90
1); [α]25D +211.3 (c 1.4, CHCl3), HPLC analysis: 90% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1.0 mL min−1, 254 nm, 8.6 min (major), 10.7 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.55–7.51 (m, 2H), 7.50–7.48 (m, 2H), 7.44–7.39 (m, 3H), 7.27–7.22 (m, 1H), 7.16 (d, J = 8.7 Hz, 2H), 7.06 (d, J = 8.1 Hz, 1H), 6.68 (d, J = 8.7 Hz, 2H), 4.67 (s, 1H), 2.38–2.29 (m, 2H), 1.20 (t, J = 6.9 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.6, 164.0, 158.1, 151.6, 135.1, 134.3, 131.6, 131.4, 130.2, 129.9, 129.4, 129.1, 128.0, 127.4, 124.3, 117.8, 116.4, 103.9, 56.1, 40.0, 29.7, 10.1. IR (KBr) ν 3010, 1856, 1648, 1556, 1262, 775. HRMS (EI) m/z: M+ Calc. for C26H19ClO4, 430.0972, Found 430.0979.
10, 1.0 mL min−1, 254 nm, 8.6 min (major), 10.7 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.55–7.51 (m, 2H), 7.50–7.48 (m, 2H), 7.44–7.39 (m, 3H), 7.27–7.22 (m, 1H), 7.16 (d, J = 8.7 Hz, 2H), 7.06 (d, J = 8.1 Hz, 1H), 6.68 (d, J = 8.7 Hz, 2H), 4.67 (s, 1H), 2.38–2.29 (m, 2H), 1.20 (t, J = 6.9 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.6, 164.0, 158.1, 151.6, 135.1, 134.3, 131.6, 131.4, 130.2, 129.9, 129.4, 129.1, 128.0, 127.4, 124.3, 117.8, 116.4, 103.9, 56.1, 40.0, 29.7, 10.1. IR (KBr) ν 3010, 1856, 1648, 1556, 1262, 775. HRMS (EI) m/z: M+ Calc. for C26H19ClO4, 430.0972, Found 430.0979.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +153.3 (c 1.1, CHCl3), HPLC analysis: 66% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 85
1); [α]25D +153.3 (c 1.1, CHCl3), HPLC analysis: 66% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 1.0 mL min−1, 254 nm, 14.9 min (major), 18.5 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.55–7.37 (m, 8H), 7.25–7.23 (m, 1H), 7.05–6.95 (m, 2H), 6.61 (d, J = 7.5 Hz, 2H), 4.64 (s, 1H), 2.35–2.30 (m, 2H), 2.22 (s, 3H), 1.19 (t, J = 6.9 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 170.3, 164.1, 158.4, 151.8, 138.1, 133.5, 131.8, 131.5, 130.0, 129.7, 129.5, 128.0, 126.0, 124.2, 117.8, 117.0, 104.2, 56.3, 40.3, 29.8, 21.1, 10.3. IR (KBr) ν 3026, 1851, 1674, 1512, 1221, 765, 565, 480. HRMS (EI) m/z: M+ Calc. for C27H22O4, 410.1518, Found 410.1523.
15, 1.0 mL min−1, 254 nm, 14.9 min (major), 18.5 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.55–7.37 (m, 8H), 7.25–7.23 (m, 1H), 7.05–6.95 (m, 2H), 6.61 (d, J = 7.5 Hz, 2H), 4.64 (s, 1H), 2.35–2.30 (m, 2H), 2.22 (s, 3H), 1.19 (t, J = 6.9 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 170.3, 164.1, 158.4, 151.8, 138.1, 133.5, 131.8, 131.5, 130.0, 129.7, 129.5, 128.0, 126.0, 124.2, 117.8, 117.0, 104.2, 56.3, 40.3, 29.8, 21.1, 10.3. IR (KBr) ν 3026, 1851, 1674, 1512, 1221, 765, 565, 480. HRMS (EI) m/z: M+ Calc. for C27H22O4, 410.1518, Found 410.1523.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +142.1 (c 1.0, CHCl3), HPLC analysis: 98% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90
1); [α]25D +142.1 (c 1.0, CHCl3), HPLC analysis: 98% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1.0 mL min−1, 254 nm, 16.9 min (major), 28.5 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.64 (d, J = 7.2 Hz, 2H), 7.50–7.32 (m, 5H), 7.16–7.09 (m, 2H), 4.27 (s, 1H), 2.32–2.24 (m, 1H), 2.04–2.02 (m, 1H), 2.02–2.00 (m, 1H), 1.80–1.08 (m, 8H), 0.87–0.85 (m, 1H). 13C NMR (300 MHz, CDCl3) δ 172.0, 160.0, 151.8, 131.7, 131.4, 130.2, 129.7, 129.5, 128.3, 127.9, 124.0, 117.7, 103.0, 49.2, 43.4, 34.8, 30.6, 30.5, 28.8, 24.8, 23.1. IR (KBr) ν 3030, 1816, 1658, 1356, 1262, 760. HRMS (EI) m/z: M+ Calc. for C24H22O4, 374.1518, Found 374.1526.
10, 1.0 mL min−1, 254 nm, 16.9 min (major), 28.5 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.64 (d, J = 7.2 Hz, 2H), 7.50–7.32 (m, 5H), 7.16–7.09 (m, 2H), 4.27 (s, 1H), 2.32–2.24 (m, 1H), 2.04–2.02 (m, 1H), 2.02–2.00 (m, 1H), 1.80–1.08 (m, 8H), 0.87–0.85 (m, 1H). 13C NMR (300 MHz, CDCl3) δ 172.0, 160.0, 151.8, 131.7, 131.4, 130.2, 129.7, 129.5, 128.3, 127.9, 124.0, 117.7, 103.0, 49.2, 43.4, 34.8, 30.6, 30.5, 28.8, 24.8, 23.1. IR (KBr) ν 3030, 1816, 1658, 1356, 1262, 760. HRMS (EI) m/z: M+ Calc. for C24H22O4, 374.1518, Found 374.1526.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +173.1 (c 1.0, CHCl3), HPLC analysis: 48% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 85
1); [α]25D +173.1 (c 1.0, CHCl3), HPLC analysis: 48% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 1.0 mL min−1, 254 nm, 12.9 min (major), 15.4 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.47 (d, J = 7.2 Hz, 1H), 7.39 (d, J = 6.9 Hz, 2H), 7.40–7.09 (m, 10H), 7.02 (d, J = 7.8 Hz, 1H), 4.33 (s, 1H), 4.04 (d, J = 15.0 Hz, 1H), 3.11 (d, J = 15.3 Hz, 1H), 2.01–1.91 (m, 1H), 1.88–1.75 (m, 1H), 0.90 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.3, 161.1, 160.9, 151.3, 135.9, 132.1, 131.1, 129.8, 129.3, 129.1, 128.8, 128.1, 127.9, 127.3, 124.1, 118.9, 118.1, 101.4, 49.6, 39.2, 37.0, 26.0, 8.7. IR (KBr) ν 2990, 1766, 1556, 1455, 1262, 779. HRMS (EI) m/z: M+ Calc. for C27H22O4, 410.1518, Found 410.1523.
15, 1.0 mL min−1, 254 nm, 12.9 min (major), 15.4 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.47 (d, J = 7.2 Hz, 1H), 7.39 (d, J = 6.9 Hz, 2H), 7.40–7.09 (m, 10H), 7.02 (d, J = 7.8 Hz, 1H), 4.33 (s, 1H), 4.04 (d, J = 15.0 Hz, 1H), 3.11 (d, J = 15.3 Hz, 1H), 2.01–1.91 (m, 1H), 1.88–1.75 (m, 1H), 0.90 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.3, 161.1, 160.9, 151.3, 135.9, 132.1, 131.1, 129.8, 129.3, 129.1, 128.8, 128.1, 127.9, 127.3, 124.1, 118.9, 118.1, 101.4, 49.6, 39.2, 37.0, 26.0, 8.7. IR (KBr) ν 2990, 1766, 1556, 1455, 1262, 779. HRMS (EI) m/z: M+ Calc. for C27H22O4, 410.1518, Found 410.1523.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +256.4 (c 1.5, CHCl3), HPLC analysis: 87% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 85
1); [α]25D +256.4 (c 1.5, CHCl3), HPLC analysis: 87% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 1.0 mL min−1, 254 nm, 12.3 min (major), 16.2 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.49–7.46(m, 3H), 7.42–7.36 (m, 1H), 7.25–7.15 (m, 6H), 7.03 (d, J = 8.1 Hz, 1H), 6.71 (d, J = 6.6 Hz, 2H), 4.64 (s, 1H), 2.39–2.31 (m, 3H), 1.18 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 170.2, 164.2, 158.3, 151.6, 142.2, 136.4, 130.0, 129.9, 129.4, 128.8, 128.6, 128.5, 128.2, 126.0, 124.0, 117.6, 116.8, 103.2, 56.8, 40.2, 29.5, 21.7, 10.1. IR (KBr) ν 3030, 1851, 1674, 1512, 1322, 775, 525, 458. HRMS (EI) m/z: M+ Calc. for C27H22O4, 410.1518, Found 410.1522.
15, 1.0 mL min−1, 254 nm, 12.3 min (major), 16.2 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.49–7.46(m, 3H), 7.42–7.36 (m, 1H), 7.25–7.15 (m, 6H), 7.03 (d, J = 8.1 Hz, 1H), 6.71 (d, J = 6.6 Hz, 2H), 4.64 (s, 1H), 2.39–2.31 (m, 3H), 1.18 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 170.2, 164.2, 158.3, 151.6, 142.2, 136.4, 130.0, 129.9, 129.4, 128.8, 128.6, 128.5, 128.2, 126.0, 124.0, 117.6, 116.8, 103.2, 56.8, 40.2, 29.5, 21.7, 10.1. IR (KBr) ν 3030, 1851, 1674, 1512, 1322, 775, 525, 458. HRMS (EI) m/z: M+ Calc. for C27H22O4, 410.1518, Found 410.1522.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +163 (c 1.0, CHCl3), HPLC analysis: 80% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90
1); [α]25D +163 (c 1.0, CHCl3), HPLC analysis: 80% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1.0 mL min−1, 254 nm, 15.2 min (major), 21.7 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.54–7.48 (m, 3H), 7.43–7.37 (m, 3H), 7.27–7.16 (m, 4H), 7.04 (d, J = 8.1 Hz, 1H), 6.71 (d, J = 6.6 Hz, 2H), 4.64 (s, 1H), 2.39–2.31 (m, 3H), 1.18 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.7, 162.7, 158.0, 151.5, 136.3, 131.4, 131.2, 130.3, 130.0, 129.4, 128.9, 128.3, 126.1, 125.9, 124.2, 117.7, 116.5, 104.6, 56.4, 40.2, 29.5, 10.1. IR (KBr) ν 3030, 1851, 1774, 1542, 1221, 785, 468. HRMS (EI) m/z: C26H19Br79O4, 474.0467, Found 474.0475, M+ Calc. for C26H19Br81O4, 476.0446, Found 474.0443.
10, 1.0 mL min−1, 254 nm, 15.2 min (major), 21.7 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.54–7.48 (m, 3H), 7.43–7.37 (m, 3H), 7.27–7.16 (m, 4H), 7.04 (d, J = 8.1 Hz, 1H), 6.71 (d, J = 6.6 Hz, 2H), 4.64 (s, 1H), 2.39–2.31 (m, 3H), 1.18 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.7, 162.7, 158.0, 151.5, 136.3, 131.4, 131.2, 130.3, 130.0, 129.4, 128.9, 128.3, 126.1, 125.9, 124.2, 117.7, 116.5, 104.6, 56.4, 40.2, 29.5, 10.1. IR (KBr) ν 3030, 1851, 1774, 1542, 1221, 785, 468. HRMS (EI) m/z: C26H19Br79O4, 474.0467, Found 474.0475, M+ Calc. for C26H19Br81O4, 476.0446, Found 474.0443.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +189.7 (c 1.3, CHCl3), HPLC analysis: 87% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90
1); [α]25D +189.7 (c 1.3, CHCl3), HPLC analysis: 87% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1.0 mL min−1, 254 nm, 16.2 min (major), 25.8 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.46–7.41 (m, 3H), 7.40–7.36 (m, 3H), 7.28–7.14 (m, 4H), 7.05 (d, J = 7.5 Hz, 1H), 6.71 (d, J = 6.6 Hz, 2H), 4.66 (s, 1H), 2.40–2.32 (m, 3H), 1.19 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.8, 162.7, 158.1, 151.5, 137.7, 136.3, 131.3, 130.1, 130.2, 129.4, 128.9, 128.4, 128.3, 125.9, 124.3, 117.7, 116.6, 104.6, 56.5, 40.2, 29.6, 10.2. IR (KBr) ν 3040, 1851, 1674, 1512, 1221, 760, 545, 480. HRMS (EI) m/z: M+ Calc. for C26H19ClO4, 430.0972, Found 430.0979.
10, 1.0 mL min−1, 254 nm, 16.2 min (major), 25.8 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.46–7.41 (m, 3H), 7.40–7.36 (m, 3H), 7.28–7.14 (m, 4H), 7.05 (d, J = 7.5 Hz, 1H), 6.71 (d, J = 6.6 Hz, 2H), 4.66 (s, 1H), 2.40–2.32 (m, 3H), 1.19 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.8, 162.7, 158.1, 151.5, 137.7, 136.3, 131.3, 130.1, 130.2, 129.4, 128.9, 128.4, 128.3, 125.9, 124.3, 117.7, 116.6, 104.6, 56.5, 40.2, 29.6, 10.2. IR (KBr) ν 3040, 1851, 1674, 1512, 1221, 760, 545, 480. HRMS (EI) m/z: M+ Calc. for C26H19ClO4, 430.0972, Found 430.0979.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +241.2 (c 1.5, CHCl3), HPLC analysis: 66% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 85
1); [α]25D +241.2 (c 1.5, CHCl3), HPLC analysis: 66% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 1.0 mL min−1, 254 nm, 9.4 min (major), 13.4 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.52–7.46 (m, 3H), 7.44–7.36 (m, 3H), 7.28–7.14 (m, 4H), 7.05 (d, J = 8.7 Hz, 1H), 6.71 (d, J = 6.6 Hz, 2H), 4.65 (s, 1H), 2.40–2.31 (m, 2H), 1.19 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.9, 162.8, 158.2, 151.6, 140.3, 137.8, 136.4, 131.5, 131.4, 130.2, 130.0, 129.5, 129.0, 128.5, 128.4, 126.0, 124.4, 117.8, 116.7, 104.7, 56.6, 40.3, 29.7, 10.3. IR (KBr) ν 3034, 1851, 1774, 1512, 1221, 790, 515, 468. HRMS (EI) m/z: M+ Calc. for C26H19ClO4, 430.0972, Found 430.0979.
15, 1.0 mL min−1, 254 nm, 9.4 min (major), 13.4 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.52–7.46 (m, 3H), 7.44–7.36 (m, 3H), 7.28–7.14 (m, 4H), 7.05 (d, J = 8.7 Hz, 1H), 6.71 (d, J = 6.6 Hz, 2H), 4.65 (s, 1H), 2.40–2.31 (m, 2H), 1.19 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.9, 162.8, 158.2, 151.6, 140.3, 137.8, 136.4, 131.5, 131.4, 130.2, 130.0, 129.5, 129.0, 128.5, 128.4, 126.0, 124.4, 117.8, 116.7, 104.7, 56.6, 40.3, 29.7, 10.3. IR (KBr) ν 3034, 1851, 1774, 1512, 1221, 790, 515, 468. HRMS (EI) m/z: M+ Calc. for C26H19ClO4, 430.0972, Found 430.0979.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +106.8 (c 1.0, CHCl3), HPLC analysis: 42% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 85
1); [α]25D +106.8 (c 1.0, CHCl3), HPLC analysis: 42% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 1.0 mL min−1, 254 nm, 19.0 min (major), 22.4 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.56 (d, J = 7.8 Hz, 1H), 7.43–7.28 (m, 3H), 7.27–7.23 (m, 5H), 7.08 (d, J = 5.1 Hz, 1H), 6.92–6.82 (br, 2H), 4.80 (s, 1H), 2.41–2.35 (t, J = 6.0 Hz, 3H), 1.25–1.23 (br, 3H). 13C NMR (300 MHz, CDCl3) δ 169.2, 159.9, 157.4, 151.6, 136.8, 133.7, 132.2, 131.2, 130.0, 129.7, 129.3, 128.9, 128.3, 127.9, 126.7, 126.2, 124.3, 116.7, 55.8, 38.8, 30.2, 10.3. IR (KBr) ν 3030, 1871, 1772, 1642, 1221, 780, 548, 468. HRMS (EI) m/z: M+ Calc. for C26H19ClO4, 430.0972, Found 430.0979.
15, 1.0 mL min−1, 254 nm, 19.0 min (major), 22.4 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.56 (d, J = 7.8 Hz, 1H), 7.43–7.28 (m, 3H), 7.27–7.23 (m, 5H), 7.08 (d, J = 5.1 Hz, 1H), 6.92–6.82 (br, 2H), 4.80 (s, 1H), 2.41–2.35 (t, J = 6.0 Hz, 3H), 1.25–1.23 (br, 3H). 13C NMR (300 MHz, CDCl3) δ 169.2, 159.9, 157.4, 151.6, 136.8, 133.7, 132.2, 131.2, 130.0, 129.7, 129.3, 128.9, 128.3, 127.9, 126.7, 126.2, 124.3, 116.7, 55.8, 38.8, 30.2, 10.3. IR (KBr) ν 3030, 1871, 1772, 1642, 1221, 780, 548, 468. HRMS (EI) m/z: M+ Calc. for C26H19ClO4, 430.0972, Found 430.0979.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +251.4 (c 1.5, CHCl3), HPLC analysis: 91% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90
1); [α]25D +251.4 (c 1.5, CHCl3), HPLC analysis: 91% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1.0 mL min−1, 254 nm, 14.8 min (major), 19.0 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.54–7.48 (m, 4H), 7.47–7.31 (m, 3H), 7.25–7.18 (m, 3H), 6.99 (d, J = 8.7 Hz, 1H), 6.76–6.73 (m, 2H), 4.62 (s, 3H), 2.36 (q, J = 7.2 Hz, 2H), 1.20 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.5, 164.5, 157.6, 150.2, 136.1, 131.6, 131.3, 130.1, 129.9, 129.2, 129.0, 128.95, 128.4, 127.9, 125.9, 119.0, 118.5, 103.1, 56.5, 40.2, 29.4, 10.1. IR (KBr) ν 3040, 1972, 1774, 1610, 1221, 780, 468. HRMS (EI) m/z: M+ Calc. for C26H19ClO4, 430.0972, Found 430.0979.
10, 1.0 mL min−1, 254 nm, 14.8 min (major), 19.0 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.54–7.48 (m, 4H), 7.47–7.31 (m, 3H), 7.25–7.18 (m, 3H), 6.99 (d, J = 8.7 Hz, 1H), 6.76–6.73 (m, 2H), 4.62 (s, 3H), 2.36 (q, J = 7.2 Hz, 2H), 1.20 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.5, 164.5, 157.6, 150.2, 136.1, 131.6, 131.3, 130.1, 129.9, 129.2, 129.0, 128.95, 128.4, 127.9, 125.9, 119.0, 118.5, 103.1, 56.5, 40.2, 29.4, 10.1. IR (KBr) ν 3040, 1972, 1774, 1610, 1221, 780, 468. HRMS (EI) m/z: M+ Calc. for C26H19ClO4, 430.0972, Found 430.0979.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]25D +213.3 (c 1.0, CHCl3), HPLC analysis: 82% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90
1); [α]25D +213.3 (c 1.0, CHCl3), HPLC analysis: 82% ee [Daicel CHIRALPAK AD-H column, 20 °C, 254 nm hexane–i-PrOH = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1.0 mL min−1, 254 nm, 16.1 min (major), 25.8 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.62 (s, 1H), 7.61–7.34 (m, 6H), 7.25–7.17 (m, 3H), 6.93 (d, J = 8.7 Hz, 1H), 6.75 (d, J = 6.0 Hz, 2H), 4.61 (s, 1H), 2.35 (q, J = 6.9 Hz, 2H), 1.19 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.5, 164.5, 157.5, 150.7, 136.0, 133.0, 132.0, 131.6, 131.3, 129.9, 128.9, 128.4, 127.9, 125.9, 119.4, 118.9, 116.5, 103.0, 56.4, 40.1, 29.4, 10.1. IR (KBr) ν 3030, 1816, 1658, 1556, 1462, 775. HRMS (EI) m/z: C26H19Br79O4, 474.0467, Found 474.0475, M+ Calc. for C26H19Br81O4, 476.0466, Found 474.0460.
10, 1.0 mL min−1, 254 nm, 16.1 min (major), 25.8 min (minor)]. 1H NMR (300 MHz, CDCl3) δ 7.62 (s, 1H), 7.61–7.34 (m, 6H), 7.25–7.17 (m, 3H), 6.93 (d, J = 8.7 Hz, 1H), 6.75 (d, J = 6.0 Hz, 2H), 4.61 (s, 1H), 2.35 (q, J = 6.9 Hz, 2H), 1.19 (t, J = 7.2 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 169.5, 164.5, 157.5, 150.7, 136.0, 133.0, 132.0, 131.6, 131.3, 129.9, 128.9, 128.4, 127.9, 125.9, 119.4, 118.9, 116.5, 103.0, 56.4, 40.1, 29.4, 10.1. IR (KBr) ν 3030, 1816, 1658, 1556, 1462, 775. HRMS (EI) m/z: C26H19Br79O4, 474.0467, Found 474.0475, M+ Calc. for C26H19Br81O4, 476.0466, Found 474.0460.
Acknowledgements
Financial support from the Ministry of Science and Technology of China (2011CB808600), National Natural Science Foundation of China (No. 21072195, 20932008), and the Chinese Academy of Sciences is gratefully acknowledged.Notes and references
- D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS.
- (a) R. D. H. Murray, J. Mendez and S. A. Brown, The Natural Coumarins: Occurrence, Chemistry, and Biochemistry, Wiley, New York, 1982 Search PubMed; (b) R. O'Kennedy and R. D. Thornes, Coumarins: Biology, Applications, and Mode of Action, Wiley, Chichester, UK, 1st edn, 1997 Search PubMed.
- For examples of enantioselective synthesis of coumarins, see: (a) E. Alden-Danforth, M. T. Scerba and T. Lectka, Org. Lett., 2008, 10, 4951 CrossRef CAS; (b) H. Alper and C. Dong, J. Org. Chem., 2004, 69, 5011 CrossRef; (c) T. Matsuda, M. Shigeno and M. Murakami, J. Am. Chem. Soc., 2007, 129, 12086 CrossRef CAS; (d) H. Lv, L. You and S. Ye, Adv. Synth. Catal., 2008, 350, 2715 CrossRef CAS; (e) X. Y. Lu and X. L. Han, Org. Lett., 2010, 12, 108 CrossRef.
- (a) H. Staudinger, Ber. Dtsch. Chem. Ges., 1905, 38, 1735 CrossRef; (b) H. Staudinger, Justus Liebigs Ann. Chem., 1907, 356, 51 CrossRef CAS.
- For review, see: (a) T. T. Tidwell, Angew. Chem., Int. Ed., 2005, 44, 5778 CrossRef CAS; (b) R. K. Orr and M. A. Calter, Tetrahedron, 2003, 59, 3545 CrossRef CAS; (c) D. H. Paull, A. Weatherwax and T. Lectka, Tetrahedron, 2009, 65, 6771 CrossRef CAS.
- For examples, see: (a) A. E. Taggi, A. M. Hafez, H. Wack, B. Young, W. J. Drury III and T. Lectka, J. Am. Chem. Soc., 2000, 122, 7831 CrossRef CAS; (b) J. Cabrera, T. Hellmuth and R. Peters, J. Org. Chem., 2010, 75, 4326–4329 CrossRef CAS; (c) K. A. Morris, K. M. Arendt, S. H. Oh and D. Romo, Org. Lett., 2010, 12, 3764–3767 CrossRef CAS; (d) M. Dochnahl and G. C. Fu, Angew. Chem., Int. Ed., 2009, 48, 2391–2393 CrossRef CAS.
- For reviews of NHC-catalyzed reactions, see: (a) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606 CrossRef CAS; (b) N. Marion, S. Díez-González and S. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 2988 CrossRef CAS; (c) J. L. Moore and T. Rovis, Top. Curr. Chem., 2010, 291, 77 CrossRef CAS.
- (a) Y.-R. Zhang, L. He, X. Wu, P.-L. Shao and S. Ye, Org. Lett., 2008, 10, 277 CrossRef CAS; (b) X.-L. Huang, L. He, P.-L. Shao and S. Ye, Angew. Chem., Int. Ed., 2009, 48, 192 CrossRef CAS; (c) P.-L. Shao, X.-Y. Chen and S. Ye, Angew. Chem., Int. Ed., 2010, 49, 8412 CrossRef CAS; (d) T.-Y. Jian, L. He, C. Tang and S. Ye, Angew. Chem., Int. Ed., 2011, 50, 9104 CrossRef CAS.
- (a) N. Duguet, C. D. Campbell, A. M. Z. Slawin and A. D. Smith, Org. Biomol. Chem., 2008, 6, 1108 RSC; (b) C. Concellón, N. Duguet and A. D. Smith, Adv. Synth. Catal., 2009, 351, 3001 CrossRef.
- (a) Y.-R. Zhang, H. Lv, D. Zhou and S. Ye, Chem.–Eur. J., 2008, 14, 8473 CrossRef CAS; (b) Bode et al. reported an NHC-catalyzed synthesis of dihydropyranone from α-chloroaldehydes and oxodienes: M. He, G. J. Uc and J. W. Bode, J. Am. Chem. Soc., 2006, 128, 15088 CrossRef CAS.
- D. Enders and J. Han, Tetrahedron: Asymmetry, 2008, 19, 1367 CrossRef CAS.
- L. He, Y.-R. Zhang, X.-L. Huang and S. Ye, Synthesis, 2008, 2825 CAS.
- The crystal was prepared from the solution of cis-3fa in DCM–ether (90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) with a trace of petroleum ether. CCDC 901312† contains the supplementary crystallographic data for this paper.
10) with a trace of petroleum ether. CCDC 901312† contains the supplementary crystallographic data for this paper. - (a) C. E. Cannizzaro, T. Strassner and K. N. Houk, J. Am. Chem. Soc., 2001, 123, 2668 CrossRef CAS; (b) C. E. Cannizzaro and K. N. Houk, J. Am. Chem. Soc., 2004, 126, 10992 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 901312. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ob26804c |
| This journal is © The Royal Society of Chemistry 2013 |


