 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Supramolecular alignment of gold nanorods via cucurbit[8]uril ternary complex formation†
Samuel T.
Jones
,
Jameel M.
Zayed
and
Oren A.
Scherman
*
Melville Laboratory for Polymer Synthesis, Department of Chemistry, University of Cambridge, Cambridge, CB2 1EW, United Kingdom. E-mail: oas23@cam.ac.uk; Fax: +44 (0)1223 334866; Tel: +44 (0)1223 334372
First published on 23rd April 2013
Abstract
We have shown, for the first time, that a three component system is capable of aligning gold nanorods (AuNRs) through supramolecular host–guest interactions leading to control over AuNR end-to-end assembly. Viologen end-functionalised AuNRs were prepared that were capable of selectively binding to a cucurbit[8]uril (CB[8]) macrocyclic host molecule. These end-functionalised AuNRs could participate in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ternary complexation with synthesised telechelic linker molecules bearing second guest moieties, in the presence of CB[8]. When the linker length was long and flexible aggregation and precipitation of AuNRs were readily observed, but with no control over the AuNR conformation. On the other hand, when the linker length was shortened thereby imparting a more rigid connection between neighboring gold nanorods, the end-to-end assembly of AuNRs was achieved. We also note that in the presence of a molecule capable of occupying the entirety of the CB[8] cavity, end-to-end assembly is not observed as the system's ability to form a 1
1 ternary complexation with synthesised telechelic linker molecules bearing second guest moieties, in the presence of CB[8]. When the linker length was long and flexible aggregation and precipitation of AuNRs were readily observed, but with no control over the AuNR conformation. On the other hand, when the linker length was shortened thereby imparting a more rigid connection between neighboring gold nanorods, the end-to-end assembly of AuNRs was achieved. We also note that in the presence of a molecule capable of occupying the entirety of the CB[8] cavity, end-to-end assembly is not observed as the system's ability to form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ternary complex is halted. Thus, the end-to-end assembly relies upon both having a relatively short and rigid linker as well as the specific, yet tuneable supramolecular 1
1 ternary complex is halted. Thus, the end-to-end assembly relies upon both having a relatively short and rigid linker as well as the specific, yet tuneable supramolecular 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ternary complexation between the three components.
1 ternary complexation between the three components.
Metal nanoparticles (NPs) have received a great deal of interest on account of their unique electronic, chemical and optical properties. Specifically, noble metals show interesting optical properties when the particle size is smaller than the incident wavelength of light.1 Incident light induces collective oscillations of the particles' conduction electrons giving rise to surface plasmon resonances (SPR). The wavelengths of noble-metal nanoparticle SPR bands are known to be highly dependant on the size, shape and composition of the NPs.2,3
Gold nanorods (AuNRs) are of interest on account of their anisotropic shape and tuneable aspect ratio, which can allow the SPR bands to be tuned over a wider range than those of non-anisotropic noble-metal NPs.4
When AuNRs are assembled, the observed SPR bands of individual rods can couple, leading to changes in the absorbance wavelength.4,5 In recent years, the aggregation and kinetics of AuNR assembly has been studied in great detail, with self-assembly techniques proving to be the most versatile.4,6,7 Due to the unique optical coupling effects achieved by end-to-end assembly of AuNRs, the ability to control such assembly is of particular importance in areas such as sensing.8,9 Additionally, there is much interest in the use of controlled assembly of nanoparticles for nano-electronic applications.10
Previous reports on supramolecular end-to-end AuNR alignment have focused on two main approaches. The first being the direct self-assembly of end-functionalised AuNRs via solvent induced aggregation of ligands bound to the ends of AuNRs.11–14 The second approach links AuNRs through two-component systems where rods are functionalised with ligands capable of binding a bridging component such as metal salts15,16 or biological motifs.7,17 For the first time, we report here a three-component system, comprising of functional AuNRs, a ditopic linker molecule, and an unique macrocycle, exploiting specific host–guest interactions. This novel approach enables greater control over the AuNR linear assembly and may allow for the formation of hierarchical assemblies that go beyond simple chains, towards elaborate structures such as hexagonal arrays of AuNRs yet unattainable using previously published techniques.
Our approach to controlling both the end-to-end alignment and the spacing between the AuNRs relies on the use of cucurbit[n]urils (CB[n], n = 5–8 of glycouril units), which are a class of rigid, barrel-shaped macrocyclic hosts with symmetric carbonyl-lined portals.18 CB[n]s are capable of forming inclusion complexes with appropriately sised guest compounds in water with high affinity (Keq ![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 1015 M−1).18–20 The larger CB[n] homologues can accommodate multiple guests inside their cavity simultaneously. In particular, CB[8] can form stable 1
1015 M−1).18–20 The larger CB[n] homologues can accommodate multiple guests inside their cavity simultaneously. In particular, CB[8] can form stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ternary complexes with an electron deficient and electron rich guest pair, such as methyl viologen (MV2+) and naphthoxy (Np) derivatives, leading to extremely high overall binding constants (Keq) of up to 1012 M−2 in water as illustrated in Fig. 1a. This has been previously exploited in the non-covalent “handcuffing” together of supramolecular polymers and block copolymers,21–23 as well as peptides and proteins.24–26 Recently, we demonstrated that CB[8] could also be used to form hybrid materials holding together gold nanoparticle–polymer composites, which could be assembled into hollow microcapsules through microfluidic techniques, highlighting the versatility and strength of this supramolecular approach towards NP assembly.27,28
1 ternary complexes with an electron deficient and electron rich guest pair, such as methyl viologen (MV2+) and naphthoxy (Np) derivatives, leading to extremely high overall binding constants (Keq) of up to 1012 M−2 in water as illustrated in Fig. 1a. This has been previously exploited in the non-covalent “handcuffing” together of supramolecular polymers and block copolymers,21–23 as well as peptides and proteins.24–26 Recently, we demonstrated that CB[8] could also be used to form hybrid materials holding together gold nanoparticle–polymer composites, which could be assembled into hollow microcapsules through microfluidic techniques, highlighting the versatility and strength of this supramolecular approach towards NP assembly.27,28
![(a) Formation of a ternary complex between methyl viologen, 2-napthol and CB[8], (b) functionalisation of AuNRs with methyl viologen moieties 1 to produce MV2+-AuNRs 2, and (c) addition of either ditopic naphthol linker (3a or 3b) (i) with CB[7], (ii) with CB[8], or (iii) with CB[8] in the presence of 4.](/image/article/2013/NR/c3nr01454a/c3nr01454a-f1.gif) | ||
| Fig. 1 (a) Formation of a ternary complex between methyl viologen, 2-napthol and CB[8], (b) functionalisation of AuNRs with methyl viologen moieties 1 to produce MV2+-AuNRs 2, and (c) addition of either ditopic naphthol linker (3a or 3b) (i) with CB[7], (ii) with CB[8], or (iii) with CB[8] in the presence of 4. | ||
In order to exploit CB[8] ternary complexation in the alignment of nanorods, AuNRs were prepared with functional moieties only at their ends. It has been shown that exclusive end-functionalisation of AuNRs is possible with the use of cetyltrimethylammonium bromide (CTAB) as a stabilising ligand, on account of strong preferential binding of CTAB molecules to the {100} side facets of the long face of AuNRs and comparatively weak binding to the curved {111} ends of the nanorods.6 The parent AuNRs were synthesised by a previously reported seed-mediated technique producing nanorods with an aspect ratio of 4 (40 nm × 10 nm) and coated with CTAB.29
A MV2+ (first guest) containing ligand (1) capable of AuNR binding through a terminal thiol group was synthesised. This ligand was then mixed with the CTAB-coated AuNRs to yield end-functionalised AuNR-MV2+ (2) as shown in Fig. 1b. These MV2+-functionalised nanorods were characterised by UV-vis spectroscopy without any changes observed in the characteristic absorbances, suggesting that no undesired aggregation of 2 took place in aqueous solution. Once functionalisation with MV2+ had been achieved, two telechelic, ditopic linker molecules with naphthol moieties at each end, but with different linker lengths (3a: n = 12 and 3b: n = 3), were prepared (see ESI†). As depicted in Fig. 1c, this allows for the alignment of the functionalised AuNRs through the formation of CB[8] ternary complexes between the MV2+ termini of two adjacent AuNRs and 3a or 3b. To ensure water solubility, which is critical for CB[n] binding, a linker with twelve ethylene glycol repeat units (3a) was designed and prepared using a bidirectional elongation route.30
Upon purification 3a was mixed with 2 in the presence of CB[8] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in an aqueous solution; precipitation occurred almost immediately, eventually forming a black precipitate and clear solution. Analysis of the black precipitate was conducted by transmission electron microscopy (TEM), which showed large aggregates of AuNRs with no control over rod conformation. Although 3a shows high water solubility and clearly aggregates 2 in the presence of CB[8], it is unable to provide enough rigidity to the system, leading to large aggregates of AuNR rather than the desired controlled end-to-end assembly of AuNRs (Fig. 4b). As the points of attachment are only at the rod ends, reducing the flexibility of the ditopic linker should promote controlled end-to-end assembly of the AuNRs.
1) in an aqueous solution; precipitation occurred almost immediately, eventually forming a black precipitate and clear solution. Analysis of the black precipitate was conducted by transmission electron microscopy (TEM), which showed large aggregates of AuNRs with no control over rod conformation. Although 3a shows high water solubility and clearly aggregates 2 in the presence of CB[8], it is unable to provide enough rigidity to the system, leading to large aggregates of AuNR rather than the desired controlled end-to-end assembly of AuNRs (Fig. 4b). As the points of attachment are only at the rod ends, reducing the flexibility of the ditopic linker should promote controlled end-to-end assembly of the AuNRs.
By reducing the length of the linker molecule it is possible to still ensure a degree of water solubility whilst decreasing the overall flexibility of the linker. In order to achieve this a triethylene glycol spacer was chosen as the hydrophilic linker between the two hydrophobic naphthoxy units (3b). Commercially available tri(ethylene glycol) di(p-toluenesulfonate) was reacted with two equivalents of 2-naphthol in the presence of anhydrous K2CO3 to arrive at 3b in 54% yield.
Upon mixing all components, 2, 3b and CB[8] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in water, no immediate change in absorbance was observed in the UV/vis spectrum; however, this is consistent with both previous observations of supramolecular AuNR alignment7 and CB[8] ternary complexation at the surface of a nanoparticle.27 To observe the formation of AuNR chains, TEM was carried out by drying the 1
1) in water, no immediate change in absorbance was observed in the UV/vis spectrum; however, this is consistent with both previous observations of supramolecular AuNR alignment7 and CB[8] ternary complexation at the surface of a nanoparticle.27 To observe the formation of AuNR chains, TEM was carried out by drying the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ternary mixture onto copper TEM grids over a few minutes. As the aqueous solution dried, the local concentration of all components increased, leading to the formation of AuNR chains, which can be seen in Fig. 2. A varying number of AuNRs per chain is observed over the whole sample with dimers (Fig. 2a and b) being most predominant and trimers (Fig. 2c and d), tetramers (Fig. 2c), pentamers (Fig. 2f) and hexamers (Fig. 2g) each being observed but to lesser degrees. It should be noted that the drying period does appear to influence the number and length of chains with longer drying times leading to samples containing more aligned AuNRs, although quantification of this trend has not yet been achieved.
1 ternary mixture onto copper TEM grids over a few minutes. As the aqueous solution dried, the local concentration of all components increased, leading to the formation of AuNR chains, which can be seen in Fig. 2. A varying number of AuNRs per chain is observed over the whole sample with dimers (Fig. 2a and b) being most predominant and trimers (Fig. 2c and d), tetramers (Fig. 2c), pentamers (Fig. 2f) and hexamers (Fig. 2g) each being observed but to lesser degrees. It should be noted that the drying period does appear to influence the number and length of chains with longer drying times leading to samples containing more aligned AuNRs, although quantification of this trend has not yet been achieved.
![TEM images depicting the alignment of AuNRs (2) with 3bvia CB[8] ternary complex formation. (a and b) Dimers of AuNRs, (c and d) trimers of AuNRs, (e) tetramer of AuNRs, (f) pentamer and dimer of AuNRs and (g) hexamer and dimer of AuNRs. Scale bars = 25 nm.](/image/article/2013/NR/c3nr01454a/c3nr01454a-f2.gif) | ||
| Fig. 2 TEM images depicting the alignment of AuNRs (2) with 3bvia CB[8] ternary complex formation. (a and b) Dimers of AuNRs, (c and d) trimers of AuNRs, (e) tetramer of AuNRs, (f) pentamer and dimer of AuNRs and (g) hexamer and dimer of AuNRs. Scale bars = 25 nm. | ||
In order to confirm that the AuNR alignment is indeed due to the formation of a CB[8] ternary complex between end-functionalised 2 and ditopic linker 3b, a control experiment with CB[7] was performed. CB[7] has a smaller annulus than CB[8] and as such is incapable of forming ternary complexes between MV2+ and Np guests.18 It can be seen that when CB[7] is used in place of CB[8] in the mixture of 2 and 3b, the AuNRs show no alignment (Fig. 4a). This observation was further verified by analysis of the TEM images (vide infra) (Fig. 3).
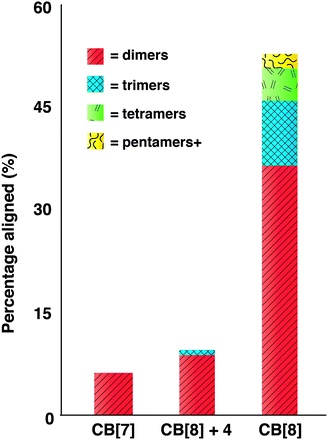 | ||
| Fig. 3 Statistical analysis of TEM images showing percentage of AuNRs aligned. | ||
Alignment of AuNRs through a supramolecular ternary complex allows for additional control over the observed morphology. The system can be disrupted via the use of a competitive guest for the CB[8] cavity, such as adamantaneamine (4). Molecule 4 has a strong binding affinity for CB[8] and is capable of displacing both the MV2+ and Np guests, inhibiting the formation of a ternary complex as shown in Fig. 1c. When 4 is added to a solution of 2, 3b and CB[8] and analysed via TEM, the alignment of 2 is inhibited (Fig. 4c). Additionally, it should be noted that in this control a large proportion of the AuNRs were seen to be aggregated side-to-side. As the CB[8] cavity is occupied by 4, it is no longer able to encapsulate the Np moieties present on the linker molecule 3b. It has been shown previously that these Np moieties are capable of incorporation into the CTAB bilayer present on the side faces of the AuNRs.31 In the presence of a ditopic Np linker (3b) and with the CB[8] cavity occupied by 4 the AuNRs are aggregated side-to-side (Fig. 4c).
![TEM images of (a) 2 in the presence of 3b and CB[7], (b) 2 in the presence of 3a and CB[8], and (c) 2 in the presence of 3b, CB[8] and 4. Scale bars = 50 nm.](/image/article/2013/NR/c3nr01454a/c3nr01454a-f4.gif) | ||
| Fig. 4 TEM images of (a) 2 in the presence of 3b and CB[7], (b) 2 in the presence of 3a and CB[8], and (c) 2 in the presence of 3b, CB[8] and 4. Scale bars = 50 nm. | ||
Statistical analysis of TEM images was undertaken to clearly show that end-to-end alignment of AuNRs is on account of CB[8] ternary complex formation between 2 and 3b and not an artifact of drying (3). From ananlysis of TEM images it can be seen that in the presence of CB[7], 93% of AuNRs show no end-to-end alignement. The remaining 7% of AuNRs are found only as dimers. This is consistent with previously reported observations for AuNR end-to-end alignment in the absence of the required stimulus.15 A low proportion of aligned AuNRs is also observed in the presence of 4 with similar percentages of AuNRs aligned as in the CB[7] control. A clear difference can be observed by TEM when the larger macrocyclic host CB[8] is used, which leads to an almost 8-fold increase in AuNRs aligned end-to-end (Fig. 3). Table S1† gives a breakdown of AuNRs that are aligned end-to-end, showing that the large majority of aligned AuNRs are dimers, likely due to the short drying times. However, unlike the very small number of aligned AuNRs observed in the CB[7] controls, larger numbers of aligned AuNRs are observed with CB[8], with nearly one-fifth of all aligned AuNRs resulting in trimers and over 10% of the remaining aligned AuNRs being found in chains of four nanorods or more. The number of AuNRs per chain is likely to be dependant upon the concentration of 3b in solution as well as the time between mixing all of the components and imaging, which has previously been shown to be of great importance.4 This observation is additionally supported by the decreasing proportions of dimers, trimers, tetramers etc. observed. Further investigations into the factors that control the degree of aggregation and ultimately chain length are currently underway in our group.
We have shown, for the first time, that a three component system is capable of aligning AuNRs through supramolecular host–guest interactions leading to control over AuNR end-to-end assembly. Viologen end-functionalised AuNRs were prepared that were capable of selectively binding to a CB[8] macrocyclic host molecule. These end-functionalised AuNRs could participate in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ternary complexation with synthesised telechelic linker molecules bearing second guest moieties, in the presence of CB[8]. When the linker length was long and flexible aggregation and precipitation of AuNRs were readily observed, but with no control over the AuNR conformation. On the other hand, when the linker length was shortened thereby imparting a more rigid connection between neighbouring gold nanorods, the end-to-end assembly of AuNRs was achieved. In the presence of a small macrocyclic homologue, CB[7], or after the addition of a competitive guest for CB[8], no alignment was observed, clearly demonstrating that ternary complex formation is critical to the alignment of AuNRs in this system. Thus, the end-to-end assembly relies upon both having a relatively short and rigid linker as well as the specific, yet tuneable supramolecular 1
1 ternary complexation with synthesised telechelic linker molecules bearing second guest moieties, in the presence of CB[8]. When the linker length was long and flexible aggregation and precipitation of AuNRs were readily observed, but with no control over the AuNR conformation. On the other hand, when the linker length was shortened thereby imparting a more rigid connection between neighbouring gold nanorods, the end-to-end assembly of AuNRs was achieved. In the presence of a small macrocyclic homologue, CB[7], or after the addition of a competitive guest for CB[8], no alignment was observed, clearly demonstrating that ternary complex formation is critical to the alignment of AuNRs in this system. Thus, the end-to-end assembly relies upon both having a relatively short and rigid linker as well as the specific, yet tuneable supramolecular 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ternary complexation between the three components.
1 ternary complexation between the three components.
References
- K. A. Willets and R. P. Van Duyne, Annu. Rev. Phys. Chem., 2007, 58, 267–297 CrossRef CAS.
- S. Eustis and M. A. El-Sayed, Chem. Soc. Rev., 2006, 35, 209–217 RSC.
- T. R. Jensen, M. D. Malinsky, C. L. Haynes and R. P. Van Duyne, J. Phys. Chem. B, 2000, 104, 10549–10556 CrossRef CAS.
- K. Liu, Z. Nie, N. Zhao, W. Li, M. Rubinstein and E. Kumacheva, Science, 2010, 329, 197–200 CrossRef CAS.
- J. Perez-Juste, I. Pastoriza-Santos, L. M. Liz-Marzan and P. Mulvaney, Coord. Chem. Rev., 2005, 249, 1870–1901 CrossRef CAS.
- D. Fava, Z. Nie, M. A. Winnik and E. Kumacheva, Adv. Mater., 2008, 20, 4318–4322 CrossRef CAS.
- K. K. Caswell, J. N. Wilson, U. H. F. Bunz and C. J. Murphy, J. Am. Chem. Soc., 2003, 125, 13914–13915 CrossRef CAS.
- G. Lu, L. Hou, T. Zhang, W. Li, J. Liu, P. Perriat and Q. Gong, J. Phys. Chem. C, 2011, 115, 22877–22885 CAS.
- L. Xu, H. Kuang, L. Wang and C. Xu, J. Mater. Chem., 2011, 21, 16759–16782 RSC.
- E. Ozbay, Science, 2006, 311, 189–193 CrossRef CAS.
- Y. Wang, A. E. DePrince, S. K. Gray, X.-M. Lin and M. Pelton, J. Phys. Chem. Lett., 2010, 1, 2692–2698 CrossRef CAS.
- Z. Nie, D. Fava, M. Rubinstein and E. Kumacheva, J. Am. Chem. Soc., 2008, 130, 3683–3689 CrossRef CAS.
- Z. Sun, W. Ni, Z. Yang, X. Kou, L. Li and J. Wang, Small, 2008, 4, 1287–1292 CrossRef CAS.
- K. G. Thomas, S. Barazzouk, B. I. Ipe, S. T. S. Joseph and P. V. Kamat, J. Phys. Chem. B, 2004, 108, 13066–13068 CrossRef CAS.
- Y.-T. Chan, S. Li, C. Moorefield, P. Wang, C. Shreiner and G. Newkome, Chem.–Eur. J., 2010, 16, 4164–4168 CrossRef CAS.
- C. Wang, Y. Chen, T. Wang, Z. Ma and Z. Su, Chem. Mater., 2007, 19, 5809–5811 CrossRef CAS.
- L. Wang, Y. Zhu, L. Xu, W. Chen, H. Kuang, L. Liu, A. Agarwal, C. Xu and N. Kotov, Angew. Chem., Int. Ed., 2010, 49, 5472–5475 CrossRef CAS.
- J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844–4870 CrossRef CAS.
- H.-J. Kim, W. S. Jeon, Y. H. Ko and K. Kim, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 5007–5011 CrossRef CAS.
- J. Kim, I. S. Jung, S. Y. Kim, E. Lee, J. K. Kang, S. Sakamoto, K. Yamaguchi and K. Kim, J. Am. Chem. Soc., 2000, 122, 540–541 CrossRef CAS.
- U. Rauwald and O. A. Scherman, Angew. Chem., Int. Ed., 2008, 47, 3950–3953 CrossRef CAS.
- X. J. Loh, J. del Barrio, P. P. C. Toh, T.-C. Lee, D. Jiao, U. Rauwald, E. A. Appel and O. A. Scherman, Biomacromolecules, 2012, 13, 84–91 CrossRef CAS.
- Y. Liu, Y. Yu, J. Gao, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2010, 49, 6576–6579 CrossRef CAS.
- M. E. Bush, N. D. Bouley and A. R. Urbach, J. Am. Chem. Soc., 2005, 127, 14511–14517 CrossRef CAS.
- H. D. Nguyen, D. T. Dang, J. L. van Dongen and L. Brunsveld, Angew. Chem., Int. Ed., 2010, 49, 895–898 CrossRef CAS.
- F. Biedermann, U. Rauwald, J. M. Zayed and O. A. Scherman, Chem. Sci., 2011, 2, 279–286 RSC.
- R. J. Coulston, S. T. Jones, T. C. Lee, E. A. Appel and O. A. Scherman, Chem. Commun., 2011, 47, 164–166 RSC.
- J. Zhang, R. J. Coulston, S. T. Jones, J. Geng, O. A. Scherman and C. Abell, Science, 2012, 335, 690–694 CrossRef CAS.
- A. Gole and C. J. Murphy, Chem. Mater., 2004, 16, 3633–3640 CrossRef CAS.
- B. A. Burkett and T. H. Chan, Synthesis, 2004, 7, 1007–1010 Search PubMed.
- A. M. Alkilany, R. L. Frey, J. L. Ferry and C. J. Murphy, Langmuir, 2008, 24, 10235–10239 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3nr01454a |
| This journal is © The Royal Society of Chemistry 2013 |
