Hot off the Press
Robert A.
Hill
and
Andrew
Sutherland
School of Chemistry, Glasgow University, Glasgow, G12 8QQ, UK. E-mail: Bob.Hill@ glasgow.ac.uk; Andrew.Sutherland@glasgow.ac.uk
First published on 1st July 2013
Abstract
A personal selection of 34 recent papers is presented covering various aspects of current developments in bioorganic chemistry and novel natural products such as nudibaccatumone from Piper nudibaccatum.
Aspergillus versicolor, isolated from the marine green alga Codium fragile, produces the alkaloid aspeverin 1 that contains a cyclic carbamate and a cyanide and shows potent bioactivities against some marine-derived organisms.1 The structure of lycospidine A 2, from Lycopodium complanatum, was confirmed by X-ray analysis.2 Lycospidine A 2 is the first example of a Lycopodium alkaloid with a five-membered ring A. A biosynthetic route to lycospidine 2 has been proposed involving proline as a precursor rather than lysine for normal Lycopodium alkaloids.
The structures of seven new alkaloids, isolated from the roots of Stemona tuberosa, were all established by X-ray analyses.3 Three of the Stemona alkaloids contain novel structural features: stemona-amine B 3 has an oxetane ring; stemona-lactam M 4 contains a 1-azabicyclo[8.2.1]tridecan-13-one unit and stemona-lactam N 5 has a 10-membered lactam. The authors speculate on the biosynthetic pathways to these alkaloids. Ternatusine A 6, from roots of Ranunculus ternatus, is the first example of an alkaloid with an epoxyoxepino[4,5-c]pyrrole ring system.4 A biosynthetic pathway to ternatusine A 6, involving two molecules of glucose, has been proposed.
Tripartin 7, the first example of a dichloromethylindanone, is a metabolite of a Streptomyces species associated with larvae of the dung beetle Copris tripartitus.5 The planar structure of tripartin 7 was confirmed by X-ray analysis together with determination of the absolute configuration. Tripartin 7 is a specific histone H3 lysine 9 demethylase (KDM4) inhibitor. Manzamenone O 8 has been isolated from a marine sponge of the genus Plakortis and is thought to be derived from three molecules of the hypothetical precursor 3,6-dioxo-4-docosenoic acid.6Myrtus communis is the source of the phloroglucinol derivative, myrtucommuacetalone 9, which has a new skeleton that was confirmed by X-ray analysis.7
The norsesquiterpenoid 10, containing a cyclobutene ring, has been reported as a constituent of the rhizomes of Alpinia japonica.8 Perovskatone A 11, from Perovskia atriplicifolia, is a C23 diterpenoid that appears to be an icetaxane diterpenoid with an expanded ring C.9 The mangrove endophytic fungus Aspergillus sp. 085242 produces the metabolites, asperterpenols A 12 and B 13, that are sesterterpenoids with a new 5/8/6/6 tetracyclic skeleton.10 The structures of asperterpenols A 12 and B 13 were confirmed by X-ray analyses.
The biosynthesis of phyllanthoid A 14, from Phyllanthus cochinchinensis, is proposed to involve a Wagner–Meerwein rearrangement of a trichin A-type limonoid to produce the 6/6/5/6 ring system.11 The structure of phyllanthoid A 14 was confirmed by X-ray analysis. Aphanamgrandiol A 15, from Aphanamixis grandifolia, is a rearranged tirucallane triterpenoid with a new skeleton.12 A biosynthetic pathway to aphanamgrandiol A 15 has been proposed. Kleinhovia hospita is the source of two cycloartane triterpenoids, kleinhospitines C 16 and D 17, that possess the 9α,10α-stereochemistry.13 A biosynthetic pathway to produce the unusual stereochemistry has been proposed.
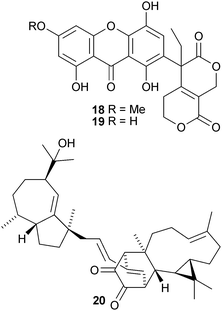
Swerpunilactones A 18 and B 19, from Swertia punicea, are the first examples of xanthones fused to a secoiridoid.14Piper nudibaccatum is the source of nudibaccatumone 20 that has a phenylpropanoid fused to two sesquiterpenoid moieties.15 Y. Asakawa and co-workers have produced an extensive review on the natural products isolated from bryophytes.16 The current knowledge of mechanism of the nitrogenase enzyme has been surveyed.17
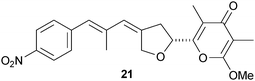
Studies by Cane and co-workers have determined the stereochemical outcome of methyl-epimerising ketoreductase domains from polyketide synthases involved in the biosynthesis of natural products such as nystatin A1.18 Incubation of (2RS)-2-methyl-3-ketopentanoyl-ACP with NADPH and various ketoreductases showed that the 2-methyl substituent had undergone enzyme-catalysed epimerisation to generate (2S,3S)-2-methyl-3-hydroxylpentanoyl-ACP (Scheme 1). Through gene deletion and complementation with a synthetic surrogate, Hertweck and co-workers have studied the factors governing the iteration processes in modular polyketide synthases that make compounds such as aureothin 21.19 For example, studies with ketosynthase domains showed that these are exclusively in charge of priming the polyketide synthase. The authors propose that these insights should allow the design of complex polyketides by pathway engineering.
 | ||
| Scheme 1 | ||
Tang and co-workers have discovered two redox enzymes, OxyS and OxyR that complete the biosynthesis of the family of tetracycline polyketide natural products that include compounds such as oxytetracycline 1 22.20 The enzyme, OxyS catalyses two sequential hydroxylations at C6 and C5, while OxyR uses F420 as a cofactor to catalyse the C5a–C11a reduction. Watanabe and co-workers have determined the role of various redox enzymes that convert prochaetoglobosin I to chaetoglobosin A 23, an inhibitor of actin polymerisation in mammalian cells.21 Using a ligD-deficient strain of Chaetomium globosum, the function of three genes encoding an FAD-dependent monooxygenase and two cyctochrome P450 oxygenases was determined.
Arnold and co-workers have designed a synthetic substrate 24 that has been used for screening the cyclisation reactions catalysed by two sesquiterpene synthase enzymes.22 This screen led to an increase in thermostability of one terpene synthase by directed evolution and improved growth and reaction conditions for the other. The authors suggest that the use of such a high throughput screen may be general and overcome challenges such as product inhibition, tolerance to organic solvents and increased activity. Using targeted gene deletions of Aspergillus terreus, a cluster of nine genes involved in the biosynthesis of acetylaranotin 25, a fungal secondary metabolite from the epipolythiodioxopiperazine family of natural products have been identified.23 Analysis of the wild-type and mutant strains allowed the isolation of seventeen natural products, nine of which are novel. Based on these studies, the authors have proposed a biosynthetic pathway for the production of acetylaranotin 25.
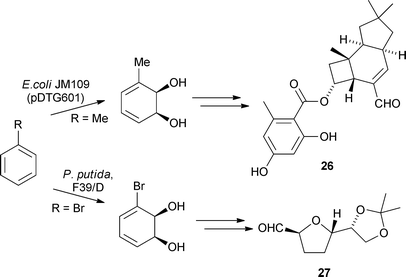
The groups of Banwell and Seoane have utilised enantiomerically pure cis-1,2-dihydrocatechols generated via a toluene dioxygenase-mediated oxidation of the corresponding benzenes, as key synthetic intermediates for the preparation of natural products (Scheme 2). Banwell and co-workers used a toluene analogue for a Diels–Alder reaction during the total synthesis of (+)-armillarivin 26,24 while Seoane and co-workers used a bromine derived system to generate the trans-tetrahydrofuran cores 27 of annonaceous acetogenins.25
 | ||
| Scheme 2 | ||
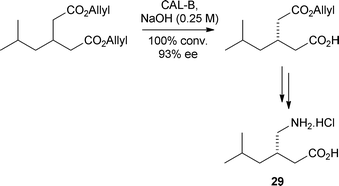
Kroutil and co-workers have used a ω-transaminase catalysed regioselective monoamination of pentadecane-2-dione as the key step for the total synthesis of the alkaloid isosolenopsin 28 (Scheme 3).26 Using a ω-transaminase originating from Arthrobacter and a co-solvent system involving DMF or n-heptane led to excellent conversions and >99% enantiomeric excess. An effective desymmetrisation of prochiral 3-alkylglutaric acid diesters was developed using immobilised lipase B from Candida antarctica.27 Desymmetrisation of substrates bearing allyl esters gave products with the highest stereoselectivity and the synthetic utility of these as synthetic intermediates was demonstrated with the total synthesis of the lipophilic GABA analogue, pregabalin® 29 (Scheme 4).
 | ||
| Scheme 3 | ||
 | ||
| Scheme 4 | ||
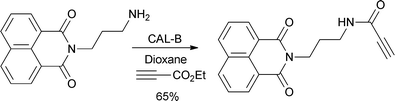
Lipase-catalysed aza-Michael addition of primary or secondary amines with acrylates and alkylacrylates was found to have excellent selectivity for the Michael addition product (e.g.Scheme 5).28 Furthermore, cross-linked enzyme aggregates, formed by the reaction of precipitated enzyme with a bifunctional agent (such as glutaraldehyde) also showed high chemoselectivity for the 1,4-addition product. Interestingly, a study has shown that lipase-catalysed reaction of amines with ethyl propiolate favoured formation of the 1,2-addition product.29 Immobilised lipase B from Candida antarctica was found to be the most effective catalyst (Scheme 6) and was also used to investigation the addition of O- and S-nucleophiles.
 | ||
| Scheme 5 | ||
 | ||
| Scheme 6 | ||
The stereoselective reduction of β-carboline imines has been achieved in high yields and excellent enantioselectivities employing cell-free extracts from red Californian earthworms (Eisenia foetida) (Scheme 7).30 This procedure was found to be more efficient than the use of whole-cell systems and provides an alternative approach to traditional catalytic asymmetric methods. The thermotolerant Kluyveromyces marxianus yeast strain has been used for the asymmetric reduction of various ketones. Fiaux and co-workers used resting cells of K. marxianus to probe the stereoselective reduction of ethyl 4,4,4-trihalide-3-oxobutanoates,31 while Vitale and co-workers employed growing cells of K. marxianus CBS 6556 for the asymmetric reduction of substituted acetophenones (Scheme 8).32
 | ||
| Scheme 7 | ||
 | ||
| Scheme 8 | ||
A new two-photon fluorescent probe 30 with near-infrared emission has been developed for detection of hydrogen sulfide in biological systems.33 The probe demonstrated significant fluorescence response for H2S compared to other reactive oxygen species and was successfully utilised in living mice. Cegelski and co-workers have shown that the natural product curcumin 31 can be used as an amyloid-specific dye in E. coli.34 Upon binding, curcumin exhibits a red-shift in absorbance and a substantial decrease in fluorescence. The authors propose that curcumin may prove useful in analysing other host-associated microbial species.
References
- N.-Y. Ji, X.-H. Liu, F.-P. Miao and M.-F. Qiao, Org. Lett., 2013, 15, 2327 CrossRef CAS.
- J.-T. Cheng, F. Liu, X.-N. Li, X.-D. Wu, L.-B. Dong, L.-Y. Peng, S.-X. Huang, J. He and Q.-S. Zhao, Org. Lett., 2013, 15, 2438 CrossRef CAS.
- Y. Hitotsuyanagi, H. Fukaya, E. Takeda, S. Matsuda, Y. Saishi, S. Zhu, K. Komatsu and K. Takeya, Tetrahedron, 2013, 69, 6297 CrossRef CAS.
- Z. Zhan, Z. Feng, Y. Yang, L. Li, J. Jiang and P. Zhang, Org. Lett., 2013, 15, 1970 CrossRef CAS.
- S.-H. Kim, S. H. Kwon, S.-H. Park, J. K. Lee, H.-S. Bang, S.-J. Nam, H. C. Kwon, J. Shin and D.-C. Oh, Org. Lett., 2013, 15, 1834 CrossRef CAS.
- N. Tanaka, M. Asai, A. Takahashi-Nakaguchi, T. Gonoi, J. Fromont and J. Kobayashi, Org. Lett., 2013, 15, 2518 CrossRef CAS.
- M. I. Choudhary, N. Khan, M. Ahmad, S. Yousuf, H.-K. Fun, S. Soomro, M. Asif, M. A. Mesaik and F. Shaheen, Org. Lett., 2013, 15, 1862 CrossRef CAS.
- Q.-M. Li, J.-G. Luo, X.-B. Wang, M.-H. Yang and L.-Y. Kong, Fitoterapia, 2013, 86, 29 CrossRef CAS.
- Z.-Y. Jiang, C.-G. Huang, H.-B. Xiong, K. Tian, W.-X. Liu, Q.-F. Hu, H.-B. Wang, G.-Y. Yang and X.-Z. Huang, Tetrahedron Lett., 2013, 54, 3886 CrossRef CAS.
- Z. Xiao, H. Huang, C. Shao, X. Xia, L. Ma, X. Huang, Y. Lu, Y. Lin, Y. Long and Z. She, Org. Lett., 2013, 15, 2522 CrossRef CAS.
- J.-Q. Zhao, Y.-M. Wang, H.-P. He, S.-H. Li, X.-N. Li, C.-R. Yang, D. Wang, H.-T. Zhu, M. Xu and Y.-J. Zhang, Org. Lett., 2013, 15, 2414 CrossRef CAS.
- Q. Zeng, B. Guan, J. Ren, C.-H. Wang, X.-R. Cheng, J.-J. Qin, S.-K. Yan, H. Z. Jin and W. D. Zhang, Fitoterapia, 2013, 86, 217 CrossRef CAS.
- C.-X. Zhou, L. Zou, L.-S. Gan and Y.-L. Cao, Org. Lett., 2013, 15, 2734 CrossRef CAS.
- H.-L. Wang, T.-W. Cao, F.-Q. Jiang, C.-A. Geng, X.-M. Zhang, X.-Y. Huang, L.-J. Wang, Kang-He, Hao-Chen, W.-J. Liang, G.-Q. Rong and J.-J. Chen, Tetrahedron Lett., 2013, 54, 2710 CrossRef CAS.
- H.-X. Liu, K. Chen, Q.-Y. Sun, F.-M. Yang, G.-W. Hu, Y.-H. Wang and C.-L. Long, J. Nat. Prod., 2013, 76, 732 CrossRef CAS.
- Y. Asakawa, A. Ludwiczuk and F. Nagashima, Phytochemistry, 2013, 91, 52 CrossRef CAS.
- B. M. Hoffman, D. Lukoyanov, D. R. Dean and L. C. Seefeldt, Acc. Chem. Res., 2013, 46, 587 CrossRef CAS.
- Y.-O. You, C. Khosla and D. E. Cane, J. Am. Chem. Soc., 2013, 135, 7406 CrossRef CAS.
- B. Busch, N. Ueberschaar, S. Behnken, Y. Sugimoto, M. Werneburg, N. Traitcheva, J. He and C. Hertweck, Angew. Chem., Int. Ed., 2013, 52, 5285 CrossRef CAS.
- P. Wang, G. Bashiri, X. Gao, M. R. Sawaya and Y. Tang, J. Am. Chem. Soc., 2013, 135, 7138 CrossRef CAS.
- K. Ishiuchi, T. Nakazawa, F. Yagishita, T. Mino, H. Noguchi, K. Hotta and K. Watanabe, J. Am. Chem. Soc., 2013, 135, 7371 CrossRef CAS.
- R. Lauchli, K. S. Rabe, K. Z. Kalbarczyk, A. Tata, T. Heel, R. Z. Kitto and F. H. Arnold, Angew. Chem., Int. Ed., 2013, 52, 5571 CrossRef CAS.
- C.-J. Guo, H.-H. Yeh, Y.-M. Chiang, J. F. Sanchez, S.-L. Chang, K. S. Bruno and C. C. C. Wang, J. Am. Chem. Soc., 2013, 135, 7205 CrossRef CAS.
- B. D. Schwartz, E. Matoušová, R. White, M. G. Banwell and A. C. Willis, Org. Lett., 2013, 15, 1934 CrossRef CAS.
- J. C. Ramos, M. Brovetto and G. A. Seoane, Org. Lett., 2013, 15, 1982 CrossRef CAS.
- R. C. Simon, C. S. Fuchs, H. Lechner, F. Zepeck and W. Kroutil, Eur. J. Org. Chem., 2013, 3397 CrossRef CAS.
- J.-H. Jung, D.-H. Yoon, P. Kang, W. K. Lee, H. Eum and H.-J. Ha, Org. Biomol. Chem., 2013, 11, 3635 CAS.
- P. Steunenberg, M. Sijm, H. Zuilhof, J. P. M. Sanders, E. L. Scott and M. C. R. Franssen, J. Org. Chem., 2013, 78, 3802 CrossRef CAS.
- S. Bonte, I. O. Ghinea, I. Baussanne, J.-P. Xuereb, R. Dinica and M. Demeunynck, Tetrahedron, 2013, 69, 5495 CrossRef CAS.
- Y. Mirabal-Gallardo, M. P. C. Soriano and L. S. Santos, Tetrahedron: Asymmetry, 2013, 24, 440 CrossRef CAS.
- S. S. S. Oliveria, L. R. S. Dias, N. C. Barbosa, M. L. Bello, F. J. M. Novaes, M. C. K. V. Ramos, F. R. A. Neto and S. B. Fiaux, Tetrahedron Lett., 2013, 54, 3067 CrossRef.
- P. Vitale, C. D'Introno, F. M. Perna, M. G. Perrone and A. Scilimati, Tetrahedron: Asymmetry, 2013, 24, 389 CrossRef CAS.
- W. Sun, J. Fan, C. Hu, J. Cao, H. Zhang, X. Xiong, J. Wang, S. Cui, S. Sun and X. Peng, Chem. Commun., 2013, 49, 3890 RSC.
- O. A. McCrate, X. Zhou and L. Cegelski, Chem. Commun., 2013, 49, 4193 RSC.
| This journal is © The Royal Society of Chemistry 2013 |