Binding of oxo-Cu2 clusters to ferric ion-binding protein A from Neisseria gonorrhoeae: a structural insight†
Abstract
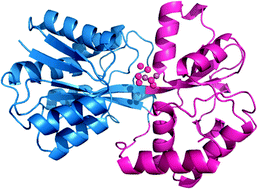
The ferric ion-binding
- This article is part of the themed collection: Metallomics in China
* Corresponding authors
a
School of Pharmacy, Second Military Medical University, Shanghai, P. R. China
E-mail:
wqzhong2002@126.com
Fax: +86 21 81871218
Tel: +86 21 81871220
b
Institute of Opto-Electronics and Institute of Molecular Science, Shanxi University, Taiyuan, P. R. China
E-mail:
wanghf@sxu.edu.cn
Fax: +86 351 7018623
Tel: +86 351 7018623
c School of Pharmacy, East China University of Science and Technology, Shanghai, P. R. China
d Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong, P. R. China
The ferric ion-binding

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
