DOI:
10.1039/C3MT00085K
(Paper)
Metallomics, 2013,
5, 1016-1024
Is aging recorded in blood Cu and Zn isotope compositions?†
Received
22nd March 2013
, Accepted 8th May 2013
First published on 10th May 2013
Abstract
Recent isotopic observations of animal samples indicate body accumulation of heavy zinc and light copper throughout life. This hypothesis has never been tested for humans, but the existence of a relationship between blood isotopic composition and age could be promising for age assessment methodologies. Dietary habits can also influence the blood zinc isotope composition, being an additional source of isotopic variation. In order to reduce this putative source of variation, we selected a population living in an isolated area (Sakha Republic, Russia) where diverse foods are of limited availability. We sampled blood from 8 male and 31 female Yakut volunteers between the ages of 18 and 74. Zinc, iron and copper were purified by liquid chromatography on ion exchange resin and their stable isotope ratios were measured using multiple-collector inductively coupled plasma mass spectrometry. According to observations of animal samples, the 66Zn/64Zn ratio increases with age. We also observe that the 65Cu/63Cu ratio decreases with age, whereas iron isotopic compositions are unrelated to age. The copper and zinc isotope compositions of the Yakut's blood are significantly lighter and heavier, respectively, than in samples of European and Japanese populations. The Yakut is a circumpolar population in which individuals have an elevated basal metabolic rate in response to cold stress. This elevated basal metabolic rate could enhance copper and zinc isotopic fractionation by accelerating the turnover of the copper and zinc stores.
Introduction
Achieving assessment of age is still a major challenge because of its implications for anthropology and forensic medicine. Methods for determining age at death are mostly based on morphological parameters such as dental development,1 dental aging,2,3 morphological changes of joints4–6 or ossification patterns.7 However, these methods are unreliable, firstly because of the variability of aging and values of bone indicators.8,9 If multifactorial and probabilistic studies show better accuracy, they can only suggest large chronological ranges.9,10 As well, new molecular aging proxies are currently being developed, based on DNA rearrangement and T-cells11,12 but they suffer from an imprecision of around ten years for best case scenarios.
Recently, it has been experimentally established that in sheep, zinc-66 (66Zn) is preferentially retained relative to 64Zn by the body, suggesting that 66Zn monotonously accumulates in the Zn stores throughout the life.13 Similarly, data obtained for sheep suggest that a possible copper (Cu) isotopic drift of the Cu stores is expected to occur throughout the life of mammals because they preferentially retained 63Cu relative to 65Cu.14 Yet, this assumption has never been tested, neither in aging animals nor in aging humans. Besides a possible “age effect” on the metal stable isotope compositions of human blood, diet is likely to produce additional isotopic variations for a given sex and age. The 56Fe/54Fe isotopic ratio of animal products is 56Fe-depleted relative to plant foodstuffs due to a marked preference for light Fe isotopes during intestinal absorption.15 The picture is more complicated for Cu and Zn isotopes because, (1) the processes at the onset of the isotopic fractionation that occur during intestinal absorption are more subtle than for Fe and, (2) the bodily isotopic fractionation among organs is more pronounced than for Fe.13 Nevertheless, a significant “diet effect” is expected to influence the isotopic compositions of Cu and Zn in human blood.16,17 In addition to a “diet effect”, it has been discovered recently a “sex effect” on the blood iron (Fe) and Cu isotope compositions.18 Firstly observed in blood, this “sex effect” has also been described for bones.19
Studies on the variations of metal stable isotope composition in human blood are still at an early stage. In more than 100 published isotopic results, most of them (>95%) document young healthy Europeans16,18,20,21 the remaining ∼5% being individuals from Japan.22,23 The present work represents an effort to document the metal isotopic variations in blood for a group of people of varying ages. Their diet is supposed to be quite uniform: as the foodstuffs are mainly non-local and Vilyuysk is quite remote24,25 (Fig. 1), the access to diversified industrialized food items is limited. We sampled blood from 39 volunteers from Vilyuysk (Sakha Republic, Russia). The volunteers only gave qualitative information on their diet. Collagen carbon and nitrogen stable isotope compositions (13C/12C and 15N/14N, respectively) are commonly used in paleoanthropology for diet reconstruction of past populations.29,30 These isotopic compositions can also be measured for modern populations26–28 in materials such as hair,26,31–33 nails34 and blood,35 as all tissues and organs are likely to be diet indicators.36,37 The relative importance of plants and animal consumption is assessed by the value of the 15N/14N ratio, because 15N accumulates up the trophic chain due to preferential excretion of 14N by animals.38 The relative influence of C3 and C4 plants on the diet is estimated using the 13C/12C ratio, C3 and C4 plants being characterized by δ13C values close to −27‰ and −13‰, respectively. The contribution of any “diet effect” to the Fe, Cu, and Zn isotope compositions was therefore tracked by measuring the carbon and nitrogen isotope compositions.
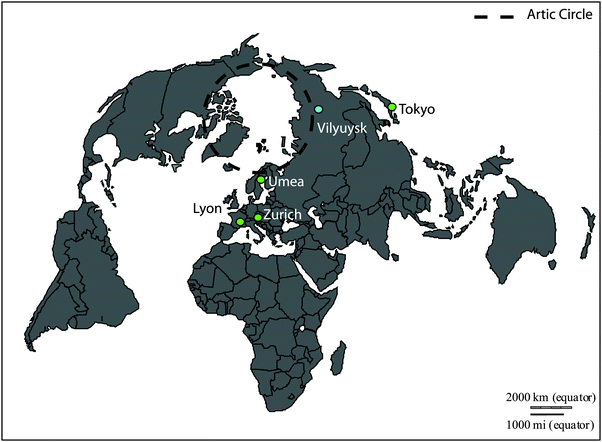 |
| | Fig. 1 Locations of the blood donor populations. Blue circle: this study (Vilyuysk, Sakha Republic, Russian Federation). Green circles: previous studies (Lyon, France; Tokyo, Japan; Umea, Sweden; Zurich, Switzerland). Blank map from Daniel Dalet (histgeo.ac-aix-marseille.fr). See text for references. | |
Materials and methods
Materials
Blood samples were collected in summer in Vacuette sampling tubes (Trace Elements grade) with informed consent in Russian and Yakut from 8 men and 31 women residing at Vilyuysk (Sakha Republic, Russia, Fig. 1). Vilyuysk is located on the Vilyuy River, a Lena's tributary (63°45′N, 121°37′E). Blood samples were also collected in Vacuette sampling tubes (Trace Elements grade) with informed consent from 5 French people residing at Lyon (Rhône-Alpes, France, Fig. 1), a city on the Rhone River. A questionnaire comprising the age of the volunteer, general information on diet, and for women, their menopausal status, was collected for both Yakut and French donors. “Postmenopausal” will hereafter refer to women who have completed menopause and “Premenopausal” will refer to women who have not. For both groups, blood collection was anonymous, and was performed in compliance with institutional guidelines. Yakut donors are between 18 and 74 years old, female donors are 44.7 ± 14.5 years old and male donors are 45.9 ± 17.2 years old. Their diet was composed of non-local foodstuffs but was complemented in high proportions by hunting, fishing and gathered foods. Samples were airlifted in ice and stored at 4 °C. They were analyzed in the Laboratoire de Géologie de Lyon, Ecole Normale Supérieure de Lyon, France.
Reagents
Macroporous anion-exchange resin AGMP-1 100–200 mesh and AG1-X8 200–400 mesh were purchased from Biorad Laboratories. Demineralized water is produced in a Millipore Synergy system. H2O2 30% Suprapur was purchased from Merck. Concentrated technical grade HCl and HNO3 also provided by Merck 64271 Darmstadt, Germany, were redistilled at low temperature in Picotrace fluoropolymer stills.
Chemical separation
The metals analyzed were separated on quartz columns containing 1.6 mL macroporous anion-exchange resin following the technique of Maréchal and Albarède.39 Samples were loaded onto the columns in 7 N HCl and rinsed in 10 mL 7 N HCl + H2O2 0.001%. Copper was eluted by 20 mL 7 N HCl + H2O2 0.001%, Fe by 10 mL 2 N HCl + H2O2 0.001%, and Zn by 10 mL 0.5 N HNO3. The Fe and Cu fractions were purified using the same method. The Zn fraction was purified using the protocol developed by Moynier et al.40 For C and N analyses, 1 mL of blood was sampled and freeze-dried. About 300 μg of freeze-dried material were weighed in tin reaction capsules and loaded into the auto-sampler of the elemental analyzer.
Isotopic measurements
Isotopic ratios of Cu and Zn were determined on a Nu-HR multiple-collector inductively-coupled plasma mass spectrometer (MC-ICPMS). Instrumental mass discrimination was corrected using elemental-doping and standard sample bracketing following the recommendations provided by Albarède et al.41 Wet plasma was used to avoid differential isotopic fractionations that occur for Zn and Cu in the membrane of the desolvating systems. Fe stable isotopes were run on a large-radius high resolution Nu-1700 MC-ICPMS operated at a resolution of 4500 as dry plasma. For both mass spectrometers, samples were introduced by free aspiration in 0.05 N sub-boiled distilled HNO3. Samples were randomized and duplicates were produced to avoid systematic errors. However, no mass spectrometer replicate has been measured for Cu isotopes because our samples do not contain enough Cu. The run conditions are listed in Table 1. The external reproducibility based on the repeated measurement of in-house standards is given in Table 2. Fe, Cu and Zn concentrations were determined using inductively-coupled plasma mass spectrometry (ICP-MS, Agilent 7500 CX). For C and N, isotopic measurements were performed by EA-pyrolysis (PyroCube analyzer, Purge and trap mode) using a system interfaced in continuous flow mode to an IRMS (GVI IsoPrime). Each sample has been measured three times and the average value is given in Table S1 (ESI†). The reproducibility on isotopic ratios is 0.2‰ for carbon, 0.1‰ for nitrogen, 0.07‰ for Cu and Zn and 0.15‰ for Fe
Table 1 Summary of analytical conditions
| Instrument |
Agilent 7500 cx |
Nu500 HR |
Nu1700 |
| RF power |
1500 W |
1350 W |
1350 W |
| Plasma gas flow rate |
15 L min−1 |
14 L min−1 |
14 L min−1 |
| Nebuliser gas flow rate |
1.1 L min−1 |
1 L min −1 |
1 L min−1 |
| Integration time |
0.1 s |
10 s per cycle |
10 s per cycle |
| Total integration time |
120 s |
20 × 2 s |
20 × 2 s |
| Collision/reaction gas |
He |
N/A |
N/A |
| Flow rate |
6 mL min−1 |
N/A |
N/A |
| Nebuliser |
GE AR351FM04 |
GE AR351FM01 |
GE AR351FM02 |
| DSN neb pressure |
N/A |
N/A |
31 psi |
| Sample cone |
Ni |
Ni |
Ni |
| Skimmer cone |
Ni |
Ni |
Ni |
Table 2 Variability of the isotopic measurements for 9 samples. SD: standard deviation of the mean and n = number of analyses
| Sample name |
Mean |
Range (‰) |
SD |
n
|
| Min |
Max |
| δ65Cu(‰) |
| GB9 |
0.13 |
−0.02 |
0.23 |
0.05 |
20 |
| Cu49n |
−0.4 |
−0.46 |
−0.33 |
0.04 |
11 |
| Cu49o |
−0.29 |
−0.36 |
−0.22 |
0.04 |
9 |
|
|
| δ56Fe(‰) |
| L1 |
−2.88 |
−2.97 |
−2.77 |
0.06 |
20 |
| 3H |
−1.91 |
−1.93 |
−1.9 |
0.01 |
5 |
|
|
| δ66Zn(‰) |
| Zn9 |
0.02 |
−0.06 |
0.07 |
0.05 |
6 |
| ZnST9 |
0.25 |
0.15 |
0.35 |
0.06 |
10 |
|
|
| δ15N(‰) |
| stdAA |
−6.48 |
−6.51 |
−6.47 |
0.01 |
10 |
|
|
| δ13C(‰) |
| stdAA |
−24.79 |
−24.96 |
−24.54 |
0.01 |
10 |
The δ notation gives the deviation in parts permil of a particular isotopic ratio relative to a specific standard. Here, 13C/12C, 15N/14N, 56Fe/54Fe, 65Cu/63Cu and 66Zn/64Zn ratios are used throughout. We adopted PDB C standard, AIR N standard, NIST SRM 976 Cu standard, JMC Lyon Zn standard, and IRMM-14 Fe standard.
Statistical methods
Shapiro–Wilk's tests were used in order to assess if data follow a normal distribution. Bilateral Student's t tests for each isotopic ratio were conducted between men and women. Concerning statistical tests involving data per age group, Kruskal–Wallis tests were performed for sets of data which did not follow a normal distribution.
Results
The complete dataset of blood isotopic results is reported in the Appendix (Table S1 and S2, ESI†), and a synthetic overview containing statistical results is given in Table 4. The range of values for duplicates is lower than the two standard-deviations (Appendix, Table S1, ESI†). We checked that isotopic fractionation is mass-dependent for all the samples, i.e. δ67Zn and δ68Zn equal 1.5 × δ66Zn and 2.0 × δ66Zn, respectively. For δ57Fe, a slope of 1.5 × δ56Fe is expected. Different slope values would indicate an instrumental mass-independent fractionation. All the data exhibit normal mass-dependent fractionation (Fig. 2).
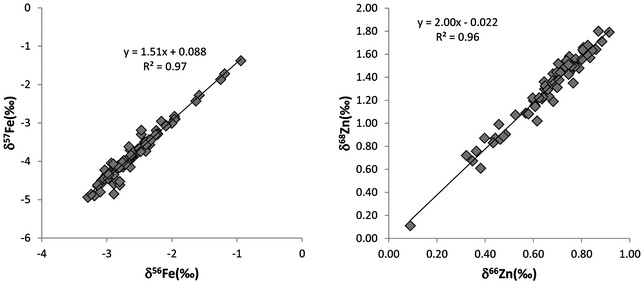 |
| | Fig. 2
Fractionation lines between different isotopes. A: δ56Fe vs. δ57Fe; B: δ68Zn vs. δ66Zn. All the samples fall on the theoretical mass-dependent fractionation lines. | |
No correlation is found between the isotopic diet proxies (δ13C and δ15N) and the metal isotope compositions (Table 3), while δ13C and δ15N seem to be weakly correlated (R² = 0.23, p = 0.002). A significant difference for δ66Zn exists between premenopausal and postmenopausal women (t test, p < 0.05). For Cu, despite the fact that the δ65Cu values of postmenopausal women cover the entire range of the observed isotopic variations, premenopausal and postmenopausal women are significantly different (t test, p < 0.005). Surprisingly, we do not notice any δ56Fe–δ65Cu sex difference even with premenopausal women as a group (Table 4). This is probably due to the low number of men that were recruited for the study. The δ65Cu and δ66Zn values are correlated negatively and positively, respectively, to age (Fig. 3) while the δ56Fe values are not correlated to age (R2 = 0.008; p = 0.59). The dependence of the δ65Cu and δ66Zn values on age is more significant when women are considered without men (R² = 0.29, p = 0.004; R² = 0.32, p = 0.001, respectively). For Zn, examination of the isotopic variations between three age groups, i.e. “young” (18–39 years old, 10 women, 3 men), “mature” (40–59 years old, 17 women, 4 men) and “old” (60–79 years old, 4 women, 1 man), reveals statistically significant differences between each age group (Fig. 4). For Cu, the isotopic values are different between “young” and “old” groups but not between “young” and “mature” groups (Fig. 4). Concerning other elements, we do not find statistical differences between the groups except for N isotope compositions between young and mature individuals, when men were taken into account.
Table 3 Correlation coefficient and associated p value for Fischer test between diet isotopic proxies and metal stable isotopes
| |
δ65Cu |
δ56Fe |
δ66Zn |
|
R² |
p
|
R²
|
p
|
R²
|
p
|
| δ13C |
0.03 |
0.28 |
0.004 |
0.67 |
0.01 |
0.54 |
| δ15N |
0.01 |
0.6 |
0.04 |
0.22 |
0.02 |
0.45 |
Table 4 Average isotope compositions in delta units (permil or ‰) and 95% range (2SD) for the isotope compositions of Fe, Cu, Zn, C and N in blood. Typical analytical uncertainties are 0.07‰ for Fe, Cu and Zn and 0.1‰ for C and N
| |
n
|
δ56Fe(‰) |
SD |
Min |
Max |
| Premenopausal women |
14 |
−2.65 |
0.33 |
−3.14 |
−1.74 |
| Postmenopausal women |
17 |
−2.60 |
0.48 |
−3.16 |
−1.13 |
| Men |
8 |
−2.63 |
0.44 |
−3.12 |
−1.96 |
|
p1 |
|
0.75 |
|
|
|
|
p2 |
|
0.9 |
|
|
|
| |
n
|
δ65Cu(‰) |
SD |
Min |
Max |
| Premenopausal women |
14 |
−0.52 |
0.22 |
−0.92 |
−0.22 |
| Postmenopausal women |
17 |
−0.84 |
0.34 |
−1.37 |
−0.31 |
| Men |
8 |
−0.74 |
0.30 |
−1.14 |
−0.32 |
|
p1 |
|
0.003 |
|
|
|
|
p2 |
|
0.04 |
|
|
|
| |
n
|
δ66Zn(‰) |
SD |
Min |
Max |
| Premenopausal women |
14 |
0.64 |
0.15 |
0.2 |
0.86 |
| Postmenopausal women |
17 |
0.76 |
0.14 |
0.36 |
0.91 |
| Men |
7 |
0.74 |
0.03 |
0.7 |
0.78 |
|
p1 |
|
0.03 |
|
|
|
|
p2 |
|
0.08 |
|
|
|
| |
n
|
δ13C(‰) |
SD |
Min |
Max |
| Premenopausal women |
14 |
−24.03 |
0.42 |
−24.81 |
−23.25 |
| Postmenopausal women |
17 |
−24.20 |
0.66 |
−25.09 |
−22.6 |
| Men |
8 |
−23.88 |
0.56 |
−24.53 |
−23.17 |
|
p1 |
|
0.41 |
|
|
|
|
p2 |
|
0.45 |
|
|
|
| |
n
|
δ15N(‰) |
SD |
Min |
Max |
| Premenopausal women |
14 |
9.74 |
0.45 |
9.18 |
11.02 |
| Postmenopausal women |
17 |
9.93 |
0.43 |
9.18 |
10.57 |
| Men |
8 |
9.77 |
0.47 |
9.07 |
10.58 |
|
p1 |
|
0.25 |
|
|
|
|
p2 |
|
0.86 |
|
|
|
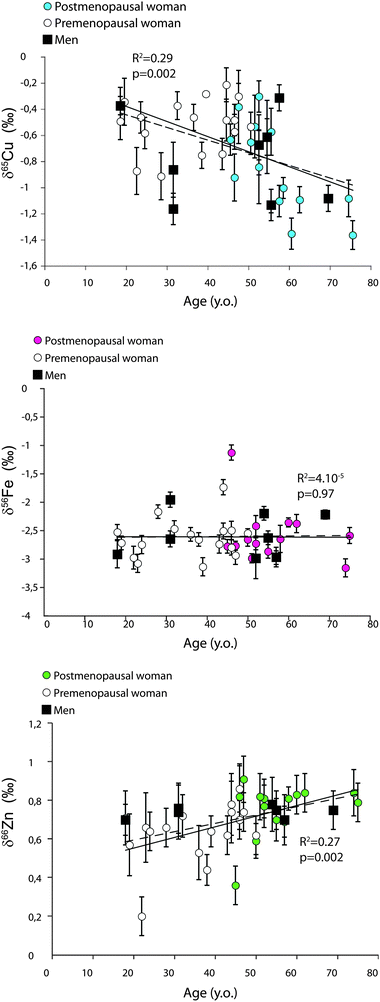 |
| | Fig. 3 Relationship between δ65Cu, δ56Fe, δ66Zn and age for Yakut population. Error bars represent the highest value between the standard deviation of sample replicates (SDsample) or that of standard replicates (SDstd). Dashed line represents the correlation between isotopic values and age for both sexes and the solid line concerns women only. | |
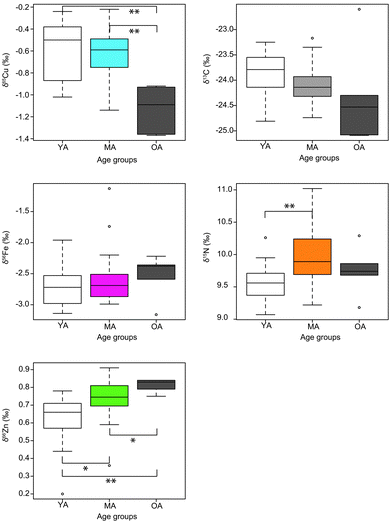 |
| | Fig. 4 Multi-isotope composition (δ65Cu, δ56Fe, δ68Zn, δ15N and δ13C) of Yakut's blood per age group. Empty boxes represent young adults (YA, <29 years old), colored boxes represent mature adults (MA, 40–59 years old), dark boxes represent the oldest adults (>59 years old). Chi-2 results for a Kruskal–Wallis test has been performed for Cu, Fe and Zn isotope composition between pairs of age groups. For two populations (k − 1) and a level of significance of 5% (±0.05), χ2 equals 3.84. * and ** is for significant results, i.e. when p < 0.05 and p < 0.005, respectively. Kruskal–Wallis tests are performed for both sexes taken together. | |
An unexpected result is the clear differences between the Yakut's δ65Cu and δ66Zn blood values and those of the reference panel (Fig. 5). The δ56Fe values in Yakut's blood are, in contrast, similar to those of the reference panel. Results of the statistical tests performed between population groups for δ65Cu, δ66Zn and δ56Fe are given Table 5. These tests were not possible for δ15N and δ13C data because only one other study has been conducted on human blood and detailed tables of results were not available.35 Moreover, we analyzed in the present study whole blood whereas Kraft et al. worked on separated clot and serum. Knowing that the amount of nitrogen and carbon contained in blood components and that clot and serum isotopic values are correlated,35 we can predict the range existing in whole blood for North American individuals with the simple equation:
| δxEwhole blood = φEclot δxEclot + φEserum δxEserum |
where E is the name of the element,
φ is the proportion of
E contained in each blood component and
x is the mass of the heavier isotope.
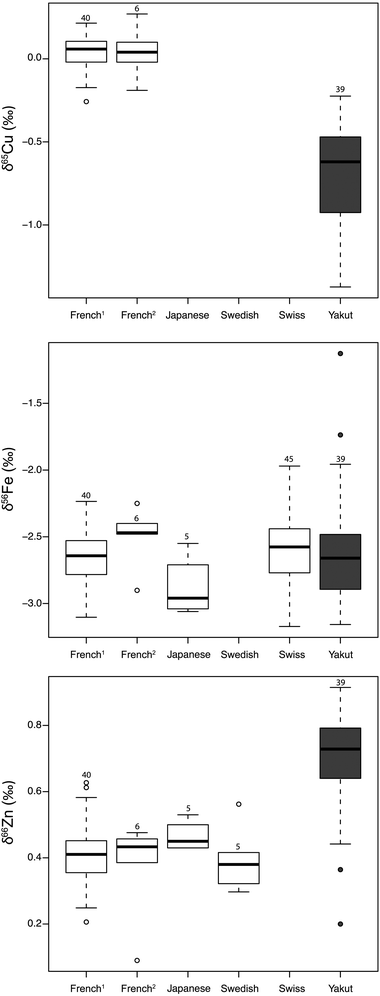 |
| | Fig. 5 Fe, Cu, and Zn isotope variations of blood for 6 populations. Data are from this study (French2, Yakut), Albarede et al., 2011 (French1), Ohno et al., 2004 (Japanese), Ohno et al., 2005 (Japanese), Stenberg et al., 2005 (Swedish) and Walczyk and von Blanckenburg, 2002 (Swiss). The box represents the 25th–75th percentiles (with the median as a bold vertical line) and the whiskers show the 10th–90th percentiles. | |
Table 5 Mean, standard deviation and Chi-2 results for a Kruskal–Wallis test performed for Cu, Fe and Zn isotope composition between population from different studies (1) Albarede et al., 2011; (2) Ohno et al. 2004 and 2005; (3) Stenberg et al., 2005; (4) Walczyk and von Blanckenburg (2002). p1 = p values for test performed between Yakut values and the one from another population. p2 = p value for test performed between French values from this study and the ones from other population
| |
δ56Fe(‰) |
| Mean |
SD |
n
|
p1 |
p2 |
| For two populations (k – 1) and a level of significance of 5% (±0.05), χ2 equals 3.84. * is for significant results, i.e. when p < 0.05. * corresponds to p < 0.05; **p < 0.005, ***p < 0.0005, ****p < 10−10. |
| Yakuts |
−2.62 |
0.4 |
39 |
— |
1.82 |
| French (this study) |
−2.5 |
0.24 |
5 |
1.82 |
— |
| French (1) |
−2.65 |
0.19 |
47 |
0.22 |
2.75 |
| Japanese (2) |
−2.91 |
0.23 |
6 |
3.50 |
5.63* |
| Swedish (3) |
— |
— |
— |
— |
— |
| Swiss (4) |
−2.59 |
0.25 |
44 |
1.72 |
1.09 |
| |
δ65Cu(‰) |
| Mean |
SD |
n
|
p1 |
p2 |
| Yakut |
−0.7 |
0.32 |
39 |
— |
13*** |
| French (this study) |
0.04 |
0.17 |
5 |
13*** |
— |
| French (1) |
0.04 |
0.1 |
47 |
62.93**** |
0.02 |
| Japanese (2) |
— |
— |
— |
— |
— |
| Swedish (3) |
— |
— |
— |
— |
— |
| Swiss (4) |
— |
— |
— |
— |
— |
| |
δ66Zn(‰) |
| Mean |
SD |
n
|
p1 |
p2 |
| Yakut |
0.68 |
0.31 |
39 |
— |
10.37** |
| French (this study) |
0.37 |
0.16 |
5 |
10.37** |
— |
| French (1) |
0.41 |
0.09 |
47 |
48.27*** |
0.02 |
| Japanese (2) |
0.45 |
0.05 |
5 |
9.65** |
0.27 |
| Swedish (3) |
0.4 |
0.1 |
5 |
10.37** |
0.27 |
| Swiss (4) |
— |
— |
— |
— |
— |
Results give a range of −16.12 to −23.31‰ for δ13C and 6.92 to 9.49‰ for δ15N (Fig. 6). Yakut values are thus distinct from those obtained in North American blood samples (Table 2, Fig. 6).
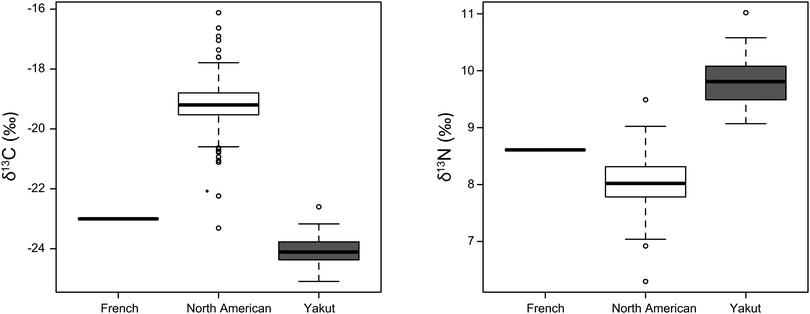 |
| | Fig. 6 N and C isotope variations of blood for 2 populations and an additional individual. Data are from this study (French, Yakut) and Kraft et al., 2008 (North American). As no table of results was available for whole blood in Kraft et al., 2008, we used the equations developed in the text in order to predict the mean, the standard deviation and the extreme values of the North American samples for δ15N and δ13C. We then choose random values, which fit with the predicted parameters. The box represents the 25th–75th percentiles (with the median as a bold vertical line) of this assumed distribution and the whiskers show the 10th–90th percentiles. | |
Discussion
Influence of age on metal stable isotopes in blood
We report significant correlations between blood Cu and Zn isotope compositions and age (Fig. 3). The trends are consistent with Balter et al.13,14 predictions based on animal experiments, i.e. a progressive light Cu and heavy Zn blood enrichment through time. The correlations between blood Cu and Zn isotope compositions and age convey a noticeable dispersion, as suggested by the weak determination coefficients (R² ≈ 0.30 for both elements). This is particularly true for the Cu isotope compositions, for which no statistical difference can be seen between “young” and “mature” groups (Fig. 4). Nevertheless, these results constitute the first demonstration that monotonous heavy Zn isotope enrichment and heavy Cu isotope depletion are observed in human blood throughout the lifespan.
Taken together, these results indicate a common mechanism of Cu and Zn isotopic fractionation amongst sheep and humans, i.e. amongst mammals. While isotopic balances between diet and feces indicate that the bulk body retains preferentially light Cu isotopes and heavy Zn isotopes, the origin of the Cu and Zn isotopic fractionations remain unknown in terms of physiological location and biochemical process. These can be unraveled with further experiments using murine models which would also present an opportunity to test whether the Cu and Zn isotope compositions of blood are recorded for bones. In such cases, Cu and Zn isotope compositions in bone could be a valuable tool for evaluating age at death in past human populations.
Given the large isotopic dispersion for each age group, aging alone cannot account for all the isotopic variability. In the state of the present results, the Cu and Zn isotope compositions cannot be used as an isotopic tool for age assessment but only as a potential supplementary proxy. The renewal of Cu and Zn in blood is linked to the erythropoietic process, and takes about 3 months. This rapid time of residence can explain the large isotopic dispersion observed for each age group in human blood. Using bone for Cu and Zn analysis might reduce the variability observed in blood because bone has a turnover of several years. On the other hand, the age determination using bone would be associated with an uncertainty at least equal to the bone turnover.
Some cohort effects can be evoked to explain the observed relationship between age and the Cu and Zn isotope composition. For instance, a difference exists between the δ15N values of young and mature Yakut (Fig. 4), suggesting that these groups do not rely on the same foodstuffs. However, this difference is not observed between young and old Yakut, and between mature and old Yakut. Keeping in mind that no relationship is observed between the δ15N and the δ65Cu and δ66Zn values, it is unlikely that dietary patterns are involved in an overall “cohort effect” on the δ65Cu and δ66Zn values. Another factor of isotopic variability could be the influence of menopause. Premenopausal women's blood is enriched in the heavy Fe isotope and 65Cu-depleted relative to the one of men, which has been indirectly attributed to high Fe and Cu requirements due to menstruations.15,18,20,42 After menopause, women's blood should become depleted in heavy Fe isotopes and 65Cu-enriched relative to premenopausal women's blood. Here we did not observe this pattern. Menopause is therefore unlikely to be involved in the relationships between age and Cu and Zn isotope compositions. The influence of physiological or lifestyle parameters such as diseases, alcohol consumption or body size cannot be evaluated because these parameters were not assessed in the questionnaires.
The Cu and Zn isotope originality of Yakut blood
The average δ65Cu and δ66Zn values in Yakut's blood are very different to those of the reference panel, which is composed of French, Swedish, Swiss and Japanese people (Fig. 1 and 5). The environmental particularity of the Vilyuysk region relies on the taiga soil, i.e. podzol. However, podzol is reported to be depleted in the light Zn isotope compared to the soil of temperate zones43 and therefore cannot account for the observed enriched heavy Zn isotope compositions. Moreover, the Yakut diet is mostly composed of non-local foodstuffs.24,25 As there is no uniformity of environmental conditions for the reference panel populations, we do not think that the isotopic originality of Yakut's blood relies on geographical or geological particularities of the soil.
Two other explanations can account for the Cu and Zn isotope originality of Yakut's blood. The first explanation is linked to a possible specific diet for Yakut. This hypothesis is supported by the Yakut's blood C and N isotope compositions, which are very different to those of North American people.35 Taking an average value of ∼1‰ for the 13C- and 15N-enrichments between diet and blood,44 the δ13C and the δ15N values of the Yakut's diet must lie around −23‰ and 11‰, respectively. This reconstructed diet is typical of a C3 environment protein-rich diet, and can include a large fraction of fish products.45,46 By comparison, the North American diet, which generates average blood δ13C and δ15N values of −19‰ and 8‰,35 respectively, is mainly based on C4-based dietary foodstuffs and is composed partly of half products of animal origin.‡ Isotopic data from animal experiments support the hypothesis of a very high proportion of animal products in the diet of the Yakut, because muscles of large mammals are 65Cu-depleted and 66Zn-enriched relative to the diet.13,17 Stating that the Cu and Zn isotopes compositions in meat are not fractionated during intestinal absorption, and that the European and North American diets are comparable, an important and enduring consumption of meat could explain why the blood δ65Cu and δ66Zn values of Yakut are 65Cu-depleted and 66Zn-enriched relative to those of Europeans. Nevertheless, the dietary hypothesis is in contradiction with the study of Van Heghe et al.,16 which shows that vegetarian blood is characterized by heavy zinc isotope enrichment in blood. However, a vegetarian diet still contains animal proteins, like dairy products and eggs. Further investigations need to be conducted on animal models or vegan populations in order to evaluate the real influence of diet on the blood metal isotope compositions.
A second explanation lies in a metabolic specificity of the Yakut compared to Japanese and European populations. Indigenous circumpolar populations, including the Yakut, exhibit an elevated body metabolic rate (BMR).24,47,48 This metabolic particularity is attributed to a physiological adaptation to cold stress,49 by up-regulation of thyroid hormones.47 If the BMR is elevated, it conveys a higher turnover of nutriment, including metals. It should lead to faster isotopic accumulation assuming equal metal intestinal absorption whatever the BMR. The idea that stable isotope turnover is linked to BMR is not new as this concept is used with doubly labeled water as an indirect calorimetric tool.50 It can be argued that Scandinavians (Uema, Fig. 1) are also a circumpolar population, although their blood Zn isotope composition is similar to that of French and Japanese populations.21 However, the cold stress is certainly higher in Vilyuysk than in Umea with temperature often plunging down to −50 °C in winter.51 This assumption requires however further investigations on circumpolar populations.
Whatever the origin of the blood Cu and Zn isotopic differences between Yakut and Europeans, our results may have potential implications for paleoanthropology and paleoecology, if they are confirmed by studies aimed at describing the fractionation of the metal isotope compositions in trophic chains.
Conclusion
The present study shows Cu and Zn isotopic specificities in Yakut blood, which we suspect to be linked to the isotope composition of the diet or to the more elevated BMRs of circumpolar populations. We also show that a relationship exists between age and blood δ65Cu and δ66Zn values, with enrichment in light Cu isotopes and heavy Zn isotopes in blood of elderly people. We suggest that this observation could be due to the preferential retention of light Cu isotopes and heavy Zn isotopes by the body. In order to confirm this assumption, the relationship between Zn and Cu isotope compositions and age should be tested in other population groups. However, this is the first time that an age-related isotopic drift is reported. We do not yet know the molecular effects of the evolution of the isotopic ratio shifts with age, but we believe that this is an avenue to explore in biomedical sciences.
Acknowledgements
This work was supported by grants from the Bullukian Foundation and the Biomérieux Research Foundation. The French archaeological Mission in Oriental Siberia (Ministère des Affaires Etrangères et Européennes, France), the North-Eastern Federal University (Yakutsk, Sakha Republic), the program HUMAD from IPEV (Institut Polaire Français Paul Emile Victor) also funded the program. Administrative and research works of this project were permitted through the program of the France Russia Associated International Laboratory (LIA COSIE number 1029), associating the North-Eastern Federal University (Yakutsk, Sakha Republic), the State medical University of Krasnoyask, the Russia Foundation for the Fundamental Research (Moscow, Russia), the University of Paul Sabatier Toulouse III (France), the University of Strasbourg I (France) and the National Centre of Scientific Research (Paris, France). We also thank Estelle Herrscher for helpful comments, as well as the two anonymous reviewers. Finally, we are very grateful to Robert C. Power for editing the English.
References
-
M. Y. Iscan and R. P. Helmer, Forensic analysis of the skull: craniofacial analysis, reconstruction, and identification, Wiley-Liss, Chichester, Brisbane, New York, 1993 Search PubMed.
- G. Gustafson, J. Am. Dent. Assoc., 1950, 41–45 Search PubMed.
- H. Lamendin, E. Baccino, J. F. Humbert, J. C. Tavernier, R. M. Nossintchouk and A. Zerilli, J. Forensic Sci., 1992, 37, 1373 CAS.
- T. W. Todd and D. W. Lyon, Am. J. Phys. Anthropol., 2005, 8, 23–45 CrossRef.
-
R. Martin, Lehrbuch der anthropologie in systematischer darstellung mit besonderer berücksichtigung der anthropologischen methoden für studierende ärtze und forschungsreisende, ed. G. Fischer, 1928, vol. 1 Search PubMed.
- C. O. Lovejoy, R. S. Meindl, T. R. Pryzbeck and R. P. Mensforth, Am. J. Phys. Anthropol., 2005, 68, 15–28 CrossRef.
-
T. W. McKern and T. D. Stewart, Skeletal age changes in young American males analysed from the standpoint of age identification, DTIC Document, 1957 Search PubMed.
- I. Hershkovitz, B. Latimer, O. Dutour, L. M. Jellema, S. Wish-Baratz, C. Rothschild and B. M. Rothschild, Am. J. Phys. Anthropol., 1997, 103, 393–399 CrossRef CAS.
- A. Schmitt, Bulletins et Mémoires de la Société d'Anthropologie de Paris, 2002, 14, 51–73 Search PubMed.
- R. S. Meindl and C. O. Lovejoy, Am. J. Phys. Anthropol., 1985, 68, 57–66 CrossRef CAS.
- C. Meissner and S. Ritz-Timme, Forensic Sci. Int., 2010, 203, 34–43 CrossRef CAS.
- D. Zubakov, F. Liu, M. C. van Zelm, J. Vermeulen, B. A. Oostra, C. M. van Duijn, G. J. Driessen, J. J. M. van Dongen, M. Kayser and A. W. Langerak, Curr. Biol., 2010, 20, R970–R971 CrossRef CAS.
- V. Balter, A. Zazzo, A. P. Moloney, F. Moynier, O. Schmidt, F. J. Monahan and F. Albarède, Rapid Commun. Mass Spectrom., 2010, 24, 605–612 CrossRef CAS.
- V. Balter and A. Zazzo, Mineral. Mag., 2011, 75, 477 Search PubMed.
- T. Walczyk and F. von Blanckenburg, Int. J. Mass Spectrom., 2005, 242, 117–134 CrossRef CAS.
- L. Van Heghe, E. Engström, I. Rodushkin, C. Cloquet and F. Vanhaecke, J. Anal. At. Spectrom., 2012, 27, 1327 RSC.
- K. Jaouen, M.-L. Pons and V. Balter, Earth Planet. Sci. Lett. DOI:10.1016/j.epsl.2013.05.037.
- F. Albarède, P. Telouk, A. Lamboux, K. Jaouen and V. Balter, Metallomics, 2011, 3, 926–933 RSC.
- K. Jaouen, V. Balter, E. Herrscher, A. Lamboux, P. Telouk and F. Albarède, Am. J. Phys. Anthropol., 2012, 148, 334–340 CrossRef.
- T. Walczyk and F. von Blanckenburg, Science, 2002, 295, 2065–2066 CrossRef CAS.
- A. Stenberg, D. Malinovsky, B. Öhlander, H. Andrén, W. Forsling, L. M. Engström, A. Wahlin, E. Engström, I. Rodushkin and D. C. Baxter, J. Trace Elem. Med. Biol., 2005, 19, 55–60 CAS.
- T. Ohno, A. Shinohara, I. Kohge, M. Chiba and T. Hirata, Anal. Sci., 2004, 20, 617–621 CrossRef CAS.
- T. Ohno, A. Shinohara, M. Chiba and T. Hirata, Anal. Sci., 2005, 21, 425–428 CrossRef CAS.
- J. J. Snodgrass, W. R. Leonard, L. A. Tarskaia, V. P. Alekseev and V. G. Krivoshapkin, Am. J. Hum. Biol., 2005, 17, 155–172 CrossRef.
- T. J. Cepon, J. J. Snodgrass, W. R. Leonard, L. A. Tarskaia, T. M. Klimova, V. I. Fedorova, M. E. Baltakhinova and V. G. Krivoshapkin, Am. J. Hum. Biol., 2011, 23, 703–709 CrossRef.
- K. J. Petzke, H. Boeing and C. C. Metges, Rapid Commun. Mass Spectrom., 2005, 19, 1392–1400 CrossRef CAS.
- A. H. Jahren and R. A. Kraft, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 17855–17860 CrossRef CAS.
- D. A. Schoeller, M. Minagawa, R. Slater and I. R. Kaplan, Ecol. Food Nutr., 1986, 18, 159–170 CrossRef CAS.
- M. P. Richards and R. E. Hedges, J. Archaeol. Sci., 1999, 26, 717–722 CrossRef.
- M. J. Schoeninger and M. J. DeNiro, Geochim. Cosmochim. Acta, 1984, 48, 625–639 CrossRef CAS.
- M. Sponheimer, T. Robinson, L. Ayliffe, B. Passey, B. Roeder, L. Shipley, E. Lopez, T. Cerling, D. Dearing and J. Ehleringer, Can. J. Zool., 2003, 81, 871–876 CrossRef CAS.
- G. E. Fahy, M. Richards, J. Riedel, J.-J. Hublin and C. Boesch, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 5829–5833 CrossRef CAS.
- V. M. Oelze, B. T. Fuller, M. P. Richards, B. Fruth, M. Surbeck, J.-J. Hublin and G. Hohmann, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 9792–9797 CrossRef CAS.
- B. T. Fuller, J. L. Fuller, D. A. Harris and R. E. Hedges, Am. J. Phys. Anthropol., 2006, 129, 279–293 CrossRef CAS.
- R. A. Kraft, A. H. Jahren and C. D. Saudek, Rapid Commun. Mass Spectrom., 2008, 22, 3683–3692 CrossRef CAS.
- J. D. Roth and K. A. Hobson, Can. J. Zool., 2000, 78, 848–852 CrossRef.
- K. A. Hobson, D. M. Schell, D. Renouf and E. Noseworthy, Can. J. Fish. Aquat. Sci., 1996, 53, 528–533 CrossRef.
- V. Balter, L. Simon, H. Fouillet and C. Lécuyer, Oecologia, 2006, 147, 212–222 CrossRef.
- C. Maréchal and F. Albarède, Geochim. Cosmochim. Acta, 2002, 66, 1499–1509 CrossRef.
- F. Moynier, F. Albarede and G. F. Herzog, Geochim. Cosmochim. Acta, 2006, 70, 6103–6117 CrossRef CAS.
- F. Albarède, P. Telouk, J. Blichert-Toft, M. Boyet, A. Agranier and B. Nelson, Geochim. Cosmochim. Acta, 2004, 68, 2725–2744 CrossRef.
- K. Hotz, P. A. Krayenbuehl and T. Walczyk, J. Biol. Inorg. Chem., 2012, 1–9 Search PubMed.
- J. Viers, P. Oliva, A. Nonell, A. Gélabert, J. E. Sonke, R. Freydier, R. Gainville and B. Dupré, Chem. Geol., 2007, 239, 124–137 CrossRef CAS.
- F. Dalerum and A. Angerbjörn, Oecologia, 2005, 144, 647–658 CrossRef CAS.
- E. Dufour, H. Bocherens and A. Mariotti, J. Archaeol. Sci., 1999, 26, 617–627 CrossRef.
- M. A. Katzenberg, H. G. McKenzie, R. J. Losey, O. I. Goriunova and A. Weber, J. Archaeol. Sci., 2012, 39, 2612–2626 CrossRef.
- W. R. Leonard, M. V. Sorensen, V. A. Galloway, G. J. Spencer, M. J. Mosher, L. Osipova and V. A. Spitsyn, Am. J. Hum. Biol., 2002, 14, 609–620 CrossRef.
- A. Rode and R. J. Shephard, Am. J. Hum. Biol., 1995, 7, 723–729 CrossRef.
- D. F. Roberts, J. Anthropol. Inst. Gt. Brit. Ireland, 1952, 169–183 Search PubMed.
- D. A. Schoeller, J. Nutr., 1988, 118, 1278–1289 CAS.
-
C. Ferret, EHESS, PhD thesis, 2006.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3mt00085k |
| ‡ According to http://www.usda.gov/factbook/chapter2.pdf. |
|
| This journal is © The Royal Society of Chemistry 2013 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article





