Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and biological evaluation of novel 2,3-dihydro-1H-1,5-benzodiazepin-2-ones; potential imaging agents of the metabotropic glutamate 2 receptor†
Lynne
Gilfillan
a,
Adele
Blair
a,
Brian J.
Morris
b,
Judith A.
Pratt
c,
Lutz
Schweiger
d,
Sally
Pimlott
e and
Andrew
Sutherland
*a
aWestCHEM, School of Chemistry, The Joseph Black Building, University of Glasgow, Glasgow, G12 8QQ, UK. E-mail: Andrew.Sutherland@glasgow.ac.uk; Fax: +44 (0)141 330 4888; Tel: +44 (0)141 330 5936
bInstitute of Neuroscience and Psychology, University of Glasgow, Glasgow, G12 8QQ, UK
cCentre for Neuroscience, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, G4 0RE, UK
dJohn Mallard Scottish PET Centre, School of Medicine and Dentistry, University of Aberdeen, Foresterhill, Aberdeen, AB25 2ZD, UK
eWest of Scotland Radionuclide Dispensary, University of Glasgow and North Glasgow University Hospital NHS Trust, Glasgow, G11 6NT, UK
First published on 29th May 2013
Abstract
A focused library of novel 2,3-dihydro-1H-1,5-benzodiazepin-2-ones containing sites for 11C-, 18F- and 123I-labelling have been prepared and evaluated against membrane expressing human recombinant metabotropic glutamate 2 receptor (mGluR2). The compounds were found to be non-competitive antagonists with nanomolar affinity. HPLC evaluation of the physiochemical properties of these compounds identified two candidates for PET and SPECT imaging of mGluR2.
Introduction
Metabotropic glutamate receptors (mGluR) are a superfamily of G-protein coupled receptors found embedded within cell membranes. In the central nervous system (CNS), they modulate L-glutamate neurotransmission and are also considered to affect dopaminergic and adrenergic neurotransmission.1 Eight members of the family of mGluRs (1–8) have been cloned and are separated into three groups based on their signal induction pathway and sequence homology.2 Group 1 receptors (mGluR1 and 5) are positively coupled to the activity of phospholipase C, whereas group II (mGluR2 and 3) and group III (mGluR4 and 6–8) receptors which have a different pharmacology, are both negatively coupled to the activity of adenyl cyclase.3The development of pharmacological agents targeting the mGluRs has been recognised as a possible approach for the treatment of various CNS disorders such as depression, anxiety and schizophrenia.4 More specifically, mGluR2/3 agonists have been shown to exhibit anxiolytic and antipsychotic properties while mGluR2/3 antagonists may be useful as anti-depressants and cognitive enhancers.4b,5,6 Recently, Woltering and co-workers initiated a programme of research to find new mGluR2/3 ligands with increased receptor selectivity and improved physiochemical properties.7 Through a random screening programme, a library of compounds based on a 2,3-dihydro-1H-1,5-benzodiazepin-2-one core 1 (Fig. 1) were identified that showed excellent affinity as non-competitive antagonists when tested against rat mGluR2 receptors. The most potent series of compounds contained 5- and 6-membered heterocyclic motifs such as 1-imidazoles, 1,2,3-triazoles and 4-pyridines at the 3′-position (R3).
 | ||
| Fig. 1 2,3-Dihydro-1H-1,5-benzodiazepin-2-one derivatives. | ||
We have a longstanding interest in developing molecular tracers for positron emission tomography (PET) and single photon emission computed tomography (SPECT) imaging of neurological receptor targets.8 Based on the 3′-imidazole derived 2,3-dihydro-1H-1,5-benzodiazepin-2-ones identified by the Woltering group, we proposed to discover novel candidates that could be used for PET and SPECT imaging of mGluR2 in schizophrenia. Such compounds would have the potential to be used for the diagnosis of schizophrenia as well as in the evaluation of new drugs and in the measurement of treatment response.
Herein, we report our studies on the design and synthesis of novel 3′-imidazole derived 2,3-dihydro-1H-1,5-benzodiazepin-2-ones bearing an iodophenyl moiety (2, 3) for SPECT imaging or methyl and fluoro groups (4, 5) for PET imaging as well as a compound with multiple labelling sites (6) which could be used for either modality (Fig. 1). We also report the biological assessment of these compounds against membrane expressing human recombinant mGluR2 as well as their suitability as neurological imaging agents by the evaluation of their physiochemical properties.
Results and discussion
We began our studies with the synthesis of 3- and 4-iodophenyl derivatives for application in SPECT imaging. Our general strategy involved the preparation of selectively protected 1,2-diaminobenzenes which could be coupled and cyclised with a β-keto ester to generate the 2,3-dihydro-1H-1,5-benzodiazepin-2-one core. Iodination would then be incorporated at the last stage allowing this step to be modified for eventual radioiodination. As such, 2-nitroaniline (7) was iodinated regioselectively using iodine monochloride9 and this was converted to Boc-protected derivative 9 using a two-step strategy involving di-protection,10 followed by selective removal of one of the Boc-protecting groups (Scheme 1). This approach was found to be more efficient for all 1,2-diaminobenzenes prepared during this study rather than direct mono-Boc protection of the aniline. A palladium(0)-mediated Suzuki–Miyaura reaction under standard conditions with 3- or 4-bromophenylboronic acid and using potassium carbonate as the base gave the corresponding bi-phenyl derivatives 10 and 11 in good overall yields. | ||
| Scheme 1 Reagents and conditions: (a) ICl, NaOAc, AcOH, 90 °C, 88%; (b) Boc2O (2.2 eq.), Et3N, DMAP (0.2 eq.), CH2Cl2, 70%; (c) TFA, CH2Cl2, 98%; (d) 3- or 4-I-PhB(OH)2, (Ph3P)4Pd (2 mol%), K2CO3, DMF–H2O, 10 (72%), 11 (78%). | ||
The aromatic core for the generation of the potential PET tracers was prepared as shown in Scheme 2. 1,5-Dichloro-2-nitro-4-(trifluoromethyl)benzene (12) was selectively aminated with ammonia to give 13 in good yield.11 Introduction of the potential PET labels by nucleophilic aromatic substitution of 13 with either methanol or 3-fluoropropan-1-ol gave alkoxy derivatives 14 and 15. Application of the two-step approach for Boc-protection of the anilines then gave compounds 16 and 17.
 | ||
| Scheme 2 Reagents and conditions: (a) NH3, 1,4-dioxane, 100 °C, 76%; (b) MeOH, KOH, DMSO, 60 °C, 97%; (c) 3-fluoropropan-1-ol, K2CO3, DMSO, 120 °C, 71%; (d) Boc2O (2.2 eq.), Et3N, DMAP (0.2 eq.), CH2Cl2, 95% (R = Me), 82% (R = 3-F-propyl); (e) TFA, CH2Cl2, 16 (100%), 17 (98%). | ||
The final compound prepared in this series required both an iodophenyl group and methoxy moiety for application in either SPECT or PET imaging, respectively. The 1,2-diaminobenzene core was initially prepared from 2-nitro-5-chloroaniline (18) which was subjected to a regioselective iodination with iodine monochloride (Scheme 3). Nucleophilic aromatic substitution at the 5-position with methanol allowed introduction of the methoxy group in excellent yield. The two-step Boc-protection sequence was then employed to give 21 and this was followed by a Suzuki–Miyaura reaction with 4-bromophenylboronic acid to give functionalised biphenyl22 in good yield over the five steps.
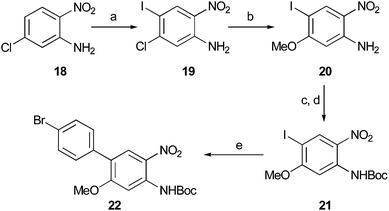 | ||
| Scheme 3 Reagents and conditions: (a) ICl, NaOAc, AcOH, 80 °C, 92%; (b) MeOH, KOH, DMSO, 60 °C, 94%; (c) Boc2O (2.2 eq.), Et3N, DMAP (0.2 eq.), CH2Cl2, 96%; (d) TFA, CH2Cl2, 100%; (e) 4-I-PhB(OH)2, (Ph3P)4Pd (2 mol%), K2CO3, DMF–H2O, 66%. | ||
The final stage of the synthesis of the selectively protected 1,2-diaminobenzenes required the reduction of the 2-nitro group. As several of these compounds (10, 11 and 22) contained labile carbon–bromine bonds, a mild procedure was required. Initial attempts using transfer hydrogenation with ammonium formate led to reduction of both the bromide and nitro groups. However, a successful transformation was identified using tin(II) chloride dihydrate which led to the reduction of 9–11, 16, 17 and 22 under basic conditions in generally high yields (Scheme 4).12 The β-keto ester fragment 30 required for coupling with the 1,2-diaminobenzenes to generate the 2,3-dihydro-1H-1,5-benzodiazepin-2-ones was prepared using a two stage approach. Methyl 3-(1′H-imidazol-1′-yl)benzoate was initially synthesised from methyl 3-aminobenzoate (29) using an imidazole ring synthesis described by Zhang and co-workers.13Claisen condensation with tert-butyl acetate gave 30 in 80% yield.14 Coupling of 30 with the 1,2-diaminobenzenes was performed under reflux to give the corresponding β-ketoamides.7 Subsequent treatment with TFA to remove the Boc-protecting group and facilitate cyclisation led to the isolation of the 2,3-dihydro-1H-1,5-benzodiazepin-2-ones in good yields over the two steps.
 | ||
| Scheme 4 Reagents and conditions: (a) SnCl2·2H2O, EtOAc/pyridine, 23 (91%), 24 (72%), 25 (63%), 26 (85%), 27 (83%), 28 (75%); (b) 40% glyoxyl, MeOH then NH4Cl, CH2O, H3PO4, 58%; (c) t-BuOAc, LiHMDS, THF, 80%; (d) Δ, toluene; (e) TFA, CH2Cl2, yields over two steps: 31 (39%), 32 (55%), 33 (66%), 4 (48%), 5 (72%), 34 (69%). | ||
The final stage of the synthesis of the SPECT compounds involved the iodination of the bromophenyl groups. Initial attempts using a direct copper catalysed halogen exchange reaction resulted in only isomerisation of the cyclic imine to the corresponding enamine.15 Instead, a palladium(0)-mediated stannylation of the bromides with hexamethylditin was followed by an oxidative iododestannylation and this gave the target iodides, 2, 3 and 6 cleanly, in good yields over the two steps (Scheme 5).
 | ||
| Scheme 5 Reagents and conditions: (a) (Me3Sn)2, (Ph3P)4Pd (10 mol%), 1,4-dioxane, 90 °C; (b) NaI, chloramine-T, EtOH, 2 (69%), 3 (42%), 6 (47%). | ||
With the series of 2,3-dihydro-1H-1,5-benzodiazepin-2-ones in hand, a functional [35S]GTPγS assay using membrane expressing human mGluR2 was optimised.16 The [35S]GTPγS assay is commonly used to determine binding to G-protein coupled receptors in vitro. On stimulation of the receptor, in this case mGluR2, with an agonist such as L-glutamate, [35S]-guanosine 5′-(γ-thio)triphosphate ([35S]GTPγS) binds irreversibly to the Gα subunit of the G-protein. This accumulation of [35S]GTPγS can be quantified by liquid scintillation analysis. Addition of a non-competitive antagonist disrupts binding of the agonist and thus, lowers accumulation of the [35S]GTPγS.
Before biological testing of the 2,3-dihydro-1H-1,5-benzodiazepin-2-ones, control experiments were performed to optimise the assay. Initially, addition of [35S]GTPγS and guanosine diphosphate to the membrane showed the expected basal binding (Fig. 2a). Stimulation with 100 μM L-glutamate resulted in a 117% increase over basal binding. Next, addition of the known competitive antagonist LY341495 (10 μM) showed the expected reduction of binding back to basal level.17 To gauge the level of non-specific binding of [35S]GTPγS, the assay was repeated with the addition of 10 μM [32S]GTPγS. This confirmed the high specificity of [35S]GTPγS for the G-protein. Finally, a further series of experiments using only 10 μM L-glutamate showed a similar level of stimulation (95%) and therefore, this concentration was used during the biological evaluation of the 2,3-dihydro-1H-1,5-benzodiazepin-2-ones (Fig. 2b).
 | ||
| Fig. 2 Graphs of control experiments with membrane expressing human mGluR2, at: (a) 100 μM L-glutamate and (b) 10 μM L-glutamate (DPM = disintegrations per minute). | ||
As a standard, commercially available Ro 64-5229 35, a known selective non-competitive mGluR2 antagonist was initially tested using the assay described above. This produced an IC50 value (533 nM) of similar magnitude to that previously reported for 35 when tested against rat mGluR2 transfected cell membranes (Table 1).18 The series of 2,3-dihydro-1H-1,5-benzodiazepin-2-ones were then evaluated. As can be observed for Table 1, all of the compounds showed excellent affinity for mGluR2 with IC50 values ranging from 89 to 133 nM. In particular, the sterically less-encumbered compounds 31 and 4 showed six times more potency than the standard, non-competitive antagonist, Ro 64-5229 35.19
Selecting potential candidates as neurological imaging agents for further development requires determination of a range of physiochemical properties. For the compound to transverse brain capillary endothelial cells and penetrate the blood brain barrier, permeability across the plasma membrane is important. Furthermore, for compounds which mainly transport across cell membranes through passive diffusion, determining the membrane partition coefficient is crucial for successful development. Based on a recently reported study of HPLC methods for determining these key physiochemical properties of imaging agents,20 the partition coefficient (log P), permeability (Pm), the membrane partition coefficient (Km) and the percentage of plasma protein binding (%PPB) of all six 2,3-dihydro-1H-1,5-benzodiazepin-2-ones were assessed (Table 2). The previously reported study performed by Tavares et al., of ten successful imaging agents established the limits of each of these parameters (log P < 4, Pm < 0.5, Km < 250, %PPB < 95%). Based on these criteria, compounds 31 and 4 were found to have the best physiochemical properties for further development. While the log P values were just above the acceptable limit, Tavares et al., do emphasise that log P is the weakest predictor of brain penetration. More importantly, 31 and 4 gave excellent results for permeability, membrane partition coefficient and percentage of plasma protein binding. Based on these results, 31 and 4 have been selected for further development as molecular imaging agents for mGluR2.
| Compound | log Pa | P m | K m | %PPBc |
|---|---|---|---|---|
| a Determined using C18 column. b Determined using immobilised artificial membrane (IAM) column. c Determined using human serum albumin (HSA) coated column. | ||||
| 31 | 4.60 | 0.51 | 218.40 | 94.92 |
| 2 | 5.73 | >2.27 | >1144.81 | 98.46 |
| 3 | 5.77 | >2.27 | >1144.81 | 98.67 |
| 4 | 4.67 | 0.44 | 176.15 | 92.41 |
| 5 | 5.06 | 0.77 | 343.73 | 95.12 |
| 6 | 5.72 | >2.13 | >1138.17 | 98.32 |
Having established 2,3-dihydro-1H-1,5-benzodiazepin-2-ones 31 and 4 as potential imaging agents for mGluR2, it was important to identify synthetic approaches for the radiolabelling of these compounds. Radioiodination of compound 31 for SPECT imaging could be envisaged using the standard two-step stannylation/oxidative iodo-destannylation already utilised for the synthesis of compounds 2, 3 and 6 (Scheme 5). Thus, application of this approach with 31 as the precursor and using [123I]- or [125I]-sodium iodide would allow introduction of radioiodine at the final step. While there are toxicity issues in using organotin compounds for the preparation of neurological SPECT imaging agents, the radioiodinated products are generally purified to a high level using HPLC.
The synthesis described for the preparation of compound 4 (Schemes 2 and 4) where the methyl group is introduced at an early stage would not be amenable to [11C]-labelling of this compound. Therefore, a synthetic route was developed to a suitable precursor that would allow [11C]-methylation as the final step (Scheme 6). Previously synthesised chloroaniline13 was subjected to a nucleophilic aromatic substitution reaction with benzyl alcohol which gave 36 in 60% yield. Boc-protection of 36 using the two-step approach followed by reduction of the nitro-group with tin(II) chloride dihydrate gave selectively protected 1,2-diaminobenzene 38 in excellent overall yield. Coupling of 38 with β-keto ester 30 and TFA mediated cyclisation gave 2,3-dihydro-1H-1,5-benzodiazepin-2-one 39 in 65% yield over the two steps. Finally, removal of the benzyl protecting group using boron tribromide gave phenol40 in 81% yield. This seven-step approach allows rapid access to a precursor which on alkylation with [11C]-methyl iodide would give the radiolabelled version of compound 4 for PET imaging. Alkylation with other groups would also result in the late-stage preparation of further non-labelled analogues in this series for biological testing.
 | ||
| Scheme 6 Reagents and conditions: (a) BnOH, KOH, n-Bu4NBr, 60 °C, 60%; (b) Boc2O (2.2 eq.), Et3N, DMAP (0.2 eq.), CH2Cl2, 98%; (c) TFA, CH2Cl2, 97%; (d) SnCl2·2H2O, EtOH, 70 °C, 98%; (e) 30, Δ, toluene; (f) TFA, CH2Cl2, 65% yields over two steps; (g) BBr3, CH2Cl2, 81%. | ||
Conclusions
In summary, six novel 2,3-dihydro-1H-1,5-benzodiazepin-2-ones bearing sites for radiolabelling have been successfully prepared using a two-stage convergent approach involving the coupling and cyclisation of a range of 1,2-diaminobenzenes with an imidazole derived β-keto ester. Biological evaluation of these compounds against membrane expressing human mGluR2 using a functional [35S]GTPγS assay showed these compounds to have excellent affinity as non-competitive antagonists for mGluR2. Evaluation of the key physiochemical properties required for a successful neurological imaging agent using established HPLC methods identified compounds 31 and 4 as potential SPECT and PET tracers for mGluR2, respectively. Work is currently underway to establish a radiosynthesis of these compounds from precursors 31 and 40 and evaluate their mGluR selectivity and biodistribution in vivo.Acknowledgements
The authors gratefully acknowledge financial support from SINAPSE (studentship to LG), the Scottish Funding Council (studentship to AB) and the University of Glasgow.Notes and references
- J.-Q. Wang and A.-L. Brownell, Curr. Med. Imaging Rev., 2007, 3, 186–205 CrossRef CAS.
- (a) F. Gasparini and W. Spooren, Curr. Neuropharmacol., 2007, 5, 187–194 CrossRef CAS; (b) R. E. Oswald, A. Ahmed, M. K. Fenwick and A. P. Loh, Curr. Drug Targets, 2007, 8, 573–582 CrossRef CAS.
- R. Pellicciari, G. Costantino, M. Marinozzi, A. Macchiarulo, E. Camaioni and B. Natalini, Farmaco, 2001, 56, 91–94 CrossRef CAS.
- (a) M. Récasens, J. Guiramand, R. Aimar, A. Abdulkarim and G. Barbanel, Curr. Drug Targets, 2007, 8, 651–681 CrossRef; (b) C. J. Swanson, M. Bures, M. P. Johnson, A.-M. Linden, J. A. Monn and D. Schoepp, Nat. Rev. Drug Discovery, 2005, 4, 131–144 CrossRef CAS.
- (a) M. P. Johnson, M. Baez, G. E. Jagdmann, Jr, T. C. Britton, T. H. Large, D. O. Callagaro, J. P. Tizzano, J. A. Monn and D. D. Schoepp, J. Med. Chem., 2003, 46, 3189–3192 CrossRef CAS; (b) S. Spinelli, T. Ballard, S. Gatti-McArthur, G. J. Richards, M. Kapps, T. Woltering, J. Wichmann, H. Stadler, J. Feldon and C. R. Pryce, Psychopharmacology, 2005, 179, 292–302 CrossRef CAS.
- (a) S. Chaki, R. Yoshikawa, S. Hirota, T. Shimazaki, M. Maeda, N. Kawashima, T. Yoshimizu, A. Yasuhara, K. Sakagami, S. Okuyama, S. Nakanishi and A. Nakazato, Neuropharmacology, 2004, 46, 457–467 CrossRef CAS; (b) G. A. Higgins, T. M. Ballard, J. N. C. Kew, J. G. Richards, J. A. Kemp, G. Adam, T. Woltering, S. Nakanishi and V. Mutel, Neuropharmacology, 2004, 46, 907–917 CrossRef CAS.
- (a) T. J. Woltering, G. Adam, A. Alanine, J. Wichmann, F. Knoflach, V. Mutel and S. Gatti, Bioorg. Med. Chem. Lett., 2007, 17, 6811–6815 CrossRef CAS; (b) T. J. Woltering, G. Adam, J. Wichmann, E. Goetschi, J. N. C. Kew, F. Knoflach, V. Mutel and S. Gatti, Bioorg. Med. Chem. Lett., 2008, 18, 1091–1095 CrossRef CAS; (c) T. J. Woltering, J. Wichmann, E. Goetschi, G. Adam, J. N. C. Kew, F. Knoflach, T. M. Ballard, J. Huwyler, V. Mutel and S. Gatti, Bioorg. Med. Chem. Lett., 2008, 18, 2725–2729 CrossRef CAS; (d) T. J. Woltering, J. Wichmann, F. Knoflach, T. M. Ballard, J. Huwyler and S. Gatti, Bioorg. Med. Chem. Lett., 2010, 20, 6969–6974 CrossRef CAS.
- (a) N. K. Jobson, A. R. Crawford, D. Dewar, S. L. Pimlott and A. Sutherland, Bioorg. Med. Chem. Lett., 2009, 19, 4996–4998 CrossRef CAS; (b) L. Stevenson, A. A. S. Tavares, A. Brunet, F. I. McGonagle, D. Dewar, S. L. Pimlott and A. Sutherland, Bioorg. Med. Chem. Lett., 2010, 20, 954–957 CrossRef CAS; (c) S. L. Pimlott and A. Sutherland, Chem. Soc. Rev., 2011, 40, 149–162 RSC; (d) A. A. Cant, R. Bhalla, S. L. Pimlott and A. Sutherland, Chem. Commun., 2012, 48, 3993–3995 RSC.
- F. Maya and J. M. Tour, Tetrahedron, 2004, 60, 81–91 CrossRef CAS.
- G. Adam, A. Alanine, E. Goetschi, V. J. Mutel and T. J. Woltering, World Patent WO01/29011, 2001.
- D. Catarzi, V. Colotta, F. Varano, F. R. Calabri, G. Filacchioni, A. Galli, C. Costagli and V. Carla, J. Med. Chem., 2004, 47, 262–272 CrossRef CAS.
- A. I. R. N. A. Barros and A. M. S. Silva, Magn. Reson. Chem., 2006, 44, 1122–1127 CrossRef CAS.
- J. Liu, J. Chen, J. Zhao, Y. Zhao, L. Li and H. Zhang, Synthesis, 2003, 2661–2666 CAS.
- A. Pendri, S. Gerritz, D. S. Dodd and C. Sun, US Pat. 20050080087, 2005.
- A. Klapars and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 14844–14845 CrossRef CAS.
- A procedure was adapted from the following: http://www.millipore.com/catalogue/item/hts146m. See ESI† for full details.
- A. E. Kingston, P. L. Ornstein, R. A. Wright, B. G. Johnson, N. G. Mayne, J. P. Burnett, R. Belagaje, S. Wu and D. D. Schoepp, Neuropharmacology, 1998, 37, 1–12 CrossRef CAS.
- Previously, using rat mGluR2 transfected cell membrane and 1S,3R-ACPD as the agonist gave an IC50 of 110 nM for Ro 64-5229 35: S. Kolczewski, G. Adam, H. Stadler, V. Mutel, J. Wichmann and T. Woltering, Bioorg. Med. Chem. Lett., 1999, 9, 2173–2176 CrossRef CAS.
- Physiochemical evaluation of compounds 3 and 6 was performed before biological testing. As the compounds did not show properties suitable for a neurological imaging agent, they were tested only twice.
- A. A. S. Tavares, J. Lewsey, D. Dewar and S. L. Pimlott, Nucl. Med. Biol., 2012, 39, 127–135 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data for all compounds synthesised, details of biological evaluation and HPLC methods, as well as NMR spectra for all new compounds. See DOI: 10.1039/c3md00110e |
| This journal is © The Royal Society of Chemistry 2013 |