Optimization of triazole-based TGR5 agonists towards orally available agents†
Kentaro
Futatsugi
*,
Kevin B.
Bahnck
,
Martin B.
Brenner
,
Joanne
Buxton
,
Janice E.
Chin
,
Steven B.
Coffey
,
Jeffrey
Dubins
,
Declan
Flynn
,
Denise
Gautreau
,
Angel
Guzman-Perez
,
John R.
Hadcock
,
David
Hepworth
,
Michael
Herr
,
Terri
Hinchey
,
Ann M.
Janssen
,
Sandra M.
Jennings
,
Wenhua
Jiao
,
Sophie Y.
Lavergne
,
Bryan
Li
,
Mei
Li
,
Michael J.
Munchhof
,
Suvi T. M.
Orr
,
David W.
Piotrowski
,
Nicole S.
Roush
,
Matthew
Sammons
,
Benjamin D.
Stevens
,
Gregory
Storer
,
Jian
Wang
,
Joseph S.
Warmus
,
Liuqing
Wei
and
Angela C.
Wolford
Pfizer Worldwide Research and Development, Eastern Point Rd, Groton, CT 06340, USA. E-mail: Kentaro.Futatsugi@pfizer.com
First published on 26th September 2012
Abstract
Reported herein is a medicinal chemistry effort towards the identification of orally available TGR5 agonist 12, which served as a dog tool compound for studies to increase confidence in this mechanism. With the challenge of striking the balance of TGR5 potency and desired clearance profile, the screening strategy as well as medicinal chemistry strategy are discussed in this article.
Introduction
TGR5 is a class A G protein-coupled receptor expressed in liver, skeletal muscle, intestine, brown adipose tissue, as well as monocytes.1–4 TGR5 agonists have been shown to stimulate secretion of glucagon-like peptide 1 (GLP1) into the blood stream via activation of receptors in the intestinal tract, suggesting the potential to provide improved glycemic control through glucose-dependent insulin secretion.5 In addition, activation of TGR5 receptors in brown adipose tissue has been proposed to increase energy expenditure through the induction of type 2 iodothyronine deiodinase (D2).6 Therefore, TGR5 agonists may provide an opportunity for treatment of diabetes with simultaneous management of glucose levels and body weight. Furthermore, recent studies suggest that the activation of TGR5 in macrophages may be of utility in atherosclerosis.7Bile acids have been reported to be endogenous agonists for TGR5, and much effort has been devoted to studying this class of compounds such as INT-777 (1, Fig. 1).4,7–10 In addition there is an increasing number of structurally diverse non-bile acid TGR5 agonists reported in the literature.4 These agonists offer the potential to (1) enable delivery of a tool compound with higher selectivity against other bile acid-mediated pathways, (2) avoid potential complications of transporter mediated pharmacokinetics, and (3) lead to a more chemically tractable medicinal chemistry program.
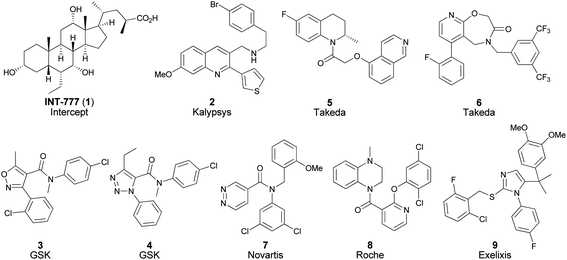 | ||
| Fig. 1 Selected TGR5 agonists reported in the literature. | ||
We wished to further investigate the therapeutic potential of non-bile acid TGR5 agonists for the treatment of metabolic diseases and for this work we sought a tool compound to perform studies for increasing confidence in the mechanism. Our key requirement for a tool compound was to achieve sufficient systemic free drug concentrations to enable rigorous testing of the effects of TGR5 activation. Despite the wide range of reported non-bile acid chemical matter selective for TGR5, our data generated from the evaluation of these ligands suggested that few of these agonists possessed sufficient potency and/or metabolic stability to meet the key requirement for a tool compound (vide infra, Fig. 2).
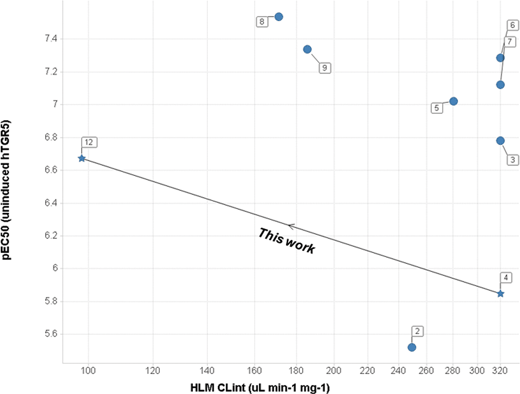 | ||
| Fig. 2 A scatter plot of representative non-bile acid TGR5 agonists reported in the literature. Y-axis: pEC50 (human TGR5 uninduced cAMP assay). X-axis: log scale plot of intrinsic clearance (CLint) in human liver microsomes (HLM). Denoted numbers correspond to the compounds 2–9 in Fig. 1, and compound 12 in Table 1. The arrow indicates the progression of the work reported herein (compounds 4 to 12). | ||
TGR5 receptor amino acid sequences for humans and dogs have been reported to be more similar than for human and rat. These differences are reflected in the divergent human and rat activity of non-bile acid agonists in their respective assays.11,12 Based on the anticipated challenge of identifying rodent tool compounds with a similar human TGR5 activity profile, we selected dog as a preclinical species, and sought to identify orally available potent agonists for both dog and human receptors. Herein we report the medicinal chemistry effort towards the identification of such an agent.
From the reported non-bile acid TGR5 agonists, triazole 4, reported by Evans and co-workers at GSK,13 was selected as an attractive starting point for generating a dog tool compound based on its favorable physico-chemical properties (molecular weight = 341, elogD14 = 2.9, and topological polar surface area = 51) and conformational rigidity (number of rotatable bonds15 = 6). It was hypothesized that the combination of these features may improve the probability of designing orally available tool compounds, by striking the balance of TGR5 activity and a clearance profile.
The screening cascade
A screening cascade was designed to facilitate our search for a tool compound that would meet our objectives. The primary criteria influencing this design were: (1) the human and dog TGR5 activities are generally aligned and (2) intrinsic clearance in human liver microsomes (HLM) is a good predictor of intrinsic clearance in dog liver microsomes (DLM). Thus, the program could use human reagents in primary screens with high confidence that both potency and metabolic stability would translate to dog. Given the known assay system-dependent effects on agonist response, we made a strategic decision to develop two TGR5 agonist assays – one with a high level of receptor expression that would provide increased sensitivity and a large dynamic range for screening compounds with widely varying potencies, and also a lower expressing system that would provide a more conservative estimate of agonist potency and intrinsic activity.16 For the system with a high level of receptor expression, a doxycycline-induced CHO cell line recombinantly expressing the human TGR5 receptor was used with generation of cAMP as the detection system (induced cAMP assay). The lower expressing system used the same cAMP detection methodology in cells expressing lower levels of either the human or dog TGR5 receptor cultured in the absence of doxycycline (uninduced cAMP assay). Concurrently, metabolic stability in HLM was monitored for improvement. Compounds with higher potency in the induced assay and lower intrinsic clearance relative to the lead 4 were next profiled in the uninduced human/dog TGR5 cAMP assays, as well as in the DLM clearance assay. With this screening cascade, we initially profiled the literature lead 4 in the human induced assay and for intrinsic clearance in HLM.Synthesis
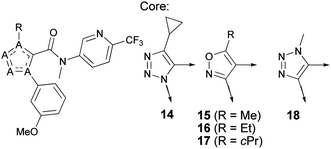
Compounds 10–14 and 18 were prepared as outlined in Scheme 1. The synthesis of 4-alkyl-1-(3-methoxyphenyl)-1H-1,2,3-triazole derivatives 10–14 started from a 1,3-dipolar cycloaddition reaction of 1-azido-3-methoxybenzene and various alkynes to afford the cycloadducts 19 after separation of their regioisomers via silica gel chromatography. Alternatively, 19 was prepared regioselectively through Cu(II)/L-proline catalyzed 1,3-dipolar cycloaddition of terminal alkynes and in situ generated 1-azido-3-methoxybenzene to obtain cycloadducts 21,17–19 followed by the lithiation–carbonylation sequence. This two-step sequence allowed delivery of the key intermediate 19 (R1 = cyclopropyl) regioselectively on a multi-gram scale without the need to synthesize organic azide. A direct amide bond formation of the ethyl ester 19 with lithium amides (Li–NMeR2), two-step ester hydrolysis–amidation sequence, or a three-step ester hydrolysis–amidation–methylation sequence furnished compounds 10–14. Compound 18 was prepared from the corresponding ester intermediate 20 by analogy with the sequence described above. The intermediate 20 was obtained in good yield through a lithiation–carbonylation sequence starting from 4-(3-methoxyphenyl)-1-methyl-1H-1,2,3-triazole, which was in turn synthesized via 1,3-dipolar cycloaddition of 1-ethynyl-3-methoxybenzene and sodium azide in the presence of a catalytic copper iodide and iodomethane in 52% yield. Isoxazole analogs 15–17 (Table 2) were prepared from the corresponding carboxylic acid intermediates.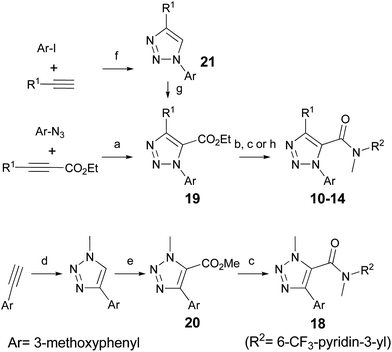 | ||
| Scheme 1 Synthesis of 10–14 and 18. Reagents and conditions: (a) 1-azido-3-methoxybenzene (1 equiv.), ethyl 2-pentynoate (2.5 equiv.), toluene, 120 °C, 22% (R1 = Et); (b) n-BuLi (1.4 or 1.5 equiv.), amine (1.2 equiv.), THF, −78 °C, 19–53%; (c) (1) NaOH or LiOH, MeOH–H2O or THF–H2O, rt to 70 °C, 34–97%; (2) (COCl)2 (1.1–3 equiv.), cat. DMF, CH2Cl2, rt, followed by HNMeR2 (1.1 or 1.4 equiv.), TEA (3 equiv.), CH2Cl2 or DME, rt or μwave, 140–190 °C, 8–66%; (d) NaN3 (1 equiv.), CuI (2.5 mol%), MeI (1 equiv.), H2O, 55 °C, 52%; (e) n-BuLi (1 equiv.), THF, −78 °C, followed by methyl chloroformate (1.2 equiv.), −78 °C to rt, 72%; (f) 3-iodoanisole (1 equiv.), ethynylcyclopropane (1 equiv.), L-proline (20 mol%), Na2CO3 (20 mol%), sodium ascorbate (20 mol%), sodium azide (1.2 equiv.), CuSO4·5H2O (10 mol%), DMSO–H2O, 65 °C, 76% (R1 = cyclopropyl); (g) n-BuLi (1.2 equiv.), THF, −78 °C to −60 °C, followed by ethyl chloroformate (1.05 equiv.), −78 °C to −60 °C, 45%; (h) (1) LiOH, MeOH–H2O, 70 °C, 97%; (2) SOCl2, cat. DMF, CH2Cl2, rt, followed by R2NH2 (1.2 equiv.), TEA (3.2 equiv.), rt; (3) iodomethane (2.7 equiv.), KOH (9 equiv.), DMSO, 50 °C, 4% (R1 = cyclopropyl, R2 = 6-trifluoromethyl-pyridin-3-yl, compound 14). | ||
Results and discussion
Compound 4 was subjected to our screening cascade, and it exhibited a modest TGR5 activity in the human induced cAMP assay. It showed high clearance in both DLM and HLM despite its moderate lipophilicity (Scheme 2).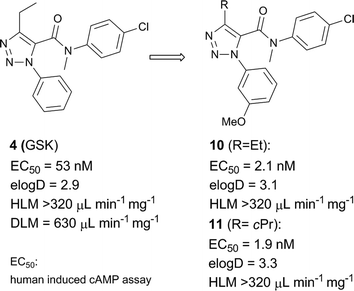 | ||
| Scheme 2 Genesis of 11 based on the literature lead 4 reported by GSK. | ||
Based on the potency/clearance profile of 4, we set the design strategy to identify in vivo tool compounds by maximizing TGR5 potency and improving clearance through reductions in overall lipophilicity and addressing metabolically weak positions of the lead. As the first step in improving the potency of 4 a 3-methoxy group was incorporated into the phenyl ring of 4. This structural modification indeed led to a significant increase in potency as previously reported in the related isoxazole series,13 although there was no detectable clearance improvement (compound 10, Scheme 2). A cyclopropyl variant of 10 was prepared in an attempt to hinder CYP (Cytochrome P450)-mediated oxidation. Unfortunately, while excellent potency was maintained as previously reported for the isoxazole series,13 no positive, observable impact on clearance was obtained. Based on this finding, we shifted attention to optimizing the electron-rich aniline region. It was postulated that introduction of an electron-withdrawing group and/or polar heteroaromatic rings may improve the CYP-mediated oxidative metabolism through reductions of overall lipophilicity and reduced oxidative liability of this region of the molecule.
As shown in Table 1, cyano analogue 12 showed reduced clearance as anticipated, while maintaining excellent potency.20 Attempts to utilize amino pyridyl groups (compounds 13 and 14) generally led to significant decreases in activity compared to that of substituted aniline derivatives, and had variable effects on clearance. For example, 2-amino pyridine analogue 13 led to a significant reduction in potency with little impact on clearance compared to compound 11. The lowest clearance in this compound set was achieved when the 4-chloro phenyl group was replaced by a 4-trifluoromethyl-3-pyridyl group, albeit at the expense of a potency reduction (compound 14). While 12 showed the best balance of TGR5 activity and in vitro clearance, it was reasoned that further optimization of the core heteroaromatic ring of 14 might allow for further increases in potency with maintenance of low clearance. Based on this hypothesis, several analogues possessing five-membered heteroaromatic ring cores were examined within a focused range of log D. Results obtained from these modifications are summarized in Table 2.
|
|
|||
|---|---|---|---|
| Cpd | Human induced EC50 ± SDa (nM) | HLM CLintb (μL min−1 mg−1) | elogD14 |
a Values are arithmetic means of values from at least three experiments.
b Intrinsic clearance determined in human liver microsomes.
c Calculated elogD using a model based on Cubist modeling methodology [Rulequest Research, www.rulequest.com]. The training set for the model was >45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 elogD values measured by the method described in ref. 14. 000 elogD values measured by the method described in ref. 14.
|
|||
| 11 | 1.9 ± 0.75 | >320 | 3.3 |
| 12 | 5.5 ± 2.2 | 98 | 2 |
| 13 | 102 ± 7.7 | 235 | (1.9)c |
| 14 | 160 ± 17 | 30 | (2.7)c |
|
|
|||
|---|---|---|---|
| Cpd | Human induced EC50 ± SDa (nM) | HLM CLintb (μL min−1 mg−1) | elogD14 |
a Values are arithmetic means of values from at least three experiments unless otherwise noted.
b Intrinsic clearance determined in human liver microsomes.
c Calculated elogD using a model based on Cubist modeling methodology [Rulequest Research, www.rulequest.com]. The training set for the model was >45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 elogD values measured by the method described in ref. 14.
d Value from single experiment. 000 elogD values measured by the method described in ref. 14.
d Value from single experiment.
|
|||
| 14 | 160 ± 17 | 30 | (2.7)c |
| 15 | 10 ± 6.4 | 60 | 2.2 |
| 16 | 11 ± 5.1 | 196 | 3.1 |
| 17 | 8.2 ± 1.9 | 135 | 3.4 |
| 18 | 135d | 43 | 1.7 |
Replacement of the triazole core by an isoxazole ring led to a 15-fold increase in potency, but at the expense of substantially increased clearance presumably as a result of increased log D (compound 14vs.17). Based on this result, it was reasoned that the reduction of log D by down-sizing the cyclopropyl group pendant to the isoxazole core may strike a better balance of potency and clearance. Consistent with the log D reduction, analogues of 17 with smaller alkyl groups at the 5 position (compounds 15 and 16) of the isoxazole core retained high potency, with reduced clearance achieved for the 5-Me analogue 15. An attempt to further reduce log D by examining isomeric triazole 18 led to the anticipated improvement in clearance but a significant decrease in potency. Based on the improvements of both TGR5 activity in the induced assay and metabolic stability in HLM compared to the literature lead 4, compounds 12 and 15 were selected for more detailed profiling in vitro (Table 3).
| Cpd | 4 | 12 | 15 |
|---|---|---|---|
| a N.D. = not determined. b Values are arithmetic means of values from at least three experiments. c Intrinsic clearance determined in human liver microsomes and dog liver microsomes. | |||
| Human induced EC50 ± SDb (nM) | 53 ± 19 | 5.5 ± 2.2 | 10 ± 6.4 |
| Human uninduced EC50 ± SDb (nM) | 1423 ± 810 | 212 ± 143 | 618 ± 288 |
| Dog uninduced EC50 ± SDb (nM) | 584 ± 156 | 196 ± 93 | 1410 ± 668 |
| HLM CLintc (μL min−1 mg−1) | >320 | 98 | 60 |
| DLM CLintc (μL min−1 mg−1) | 620 | 79 | N.D. |
| elogD14 | 2.9 | 2 | 2.2 |
Compound 12 showed improved potency over 4 in both human and dog uninduced TGR5 assays. Consistent with HLM clearance data, 12 showed reduced clearance in DLM compared to 4. Thus, compound 12 was selected for detailed determination of the pharmacokinetic properties in dog (Table 4).
| CL p (mL min−1 kg−1) | V dss (L kg−1) | T 1/2 (h) | C max (μM) | T max (h) | %F | f u, plasmab |
|---|---|---|---|---|---|---|
a Pharmacokinetic parameters as geometric mean of n = 3 (iv) or n = 2 (po) animals per group. iv dose = 1 mg kg−1 (formulation: polyethylene glycol-400 and a 30% sulfobutylether-β-cyclodextrin (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, v/v)); po dose = 3 mg kg−1 (formulation: 0.5% (w/v) methylcellulose suspension).
b Unbound dog plasma fraction determined by equilibrium dialysis. 60, v/v)); po dose = 3 mg kg−1 (formulation: 0.5% (w/v) methylcellulose suspension).
b Unbound dog plasma fraction determined by equilibrium dialysis.
|
||||||
| 22 | 4.6 | 4.7 | 0.51 | 0.25 | 17 | 0.27 |
Compound 12 showed moderate to high plasma clearance reflected in the turnover observed in the DLM system. Due to its moderate to high volume of distribution, however, a reasonable half-life could be achieved. Gratifyingly, a modest but acceptable bioavailability was obtained after oral administration of 12. While not representing an ideal pharmacokinetic profile, the achievable exposure for this agent following oral dosing was sufficient to consider it as a tool compound for further characterization of the mechanism.
To increase confidence in functional potency determination by the primary cAMP assays, compound 12 was evaluated in a human ex vivo whole blood assay. A dose dependent suppression of TNFα release was demonstrated with compound 12 after a 30 minute compound pretreatment and stimulation of human whole blood with LPS (lipopolysaccharides at 10 ng mL−1 for 4–5 hours stimulation). The data indicate that the uninduced EC50 in the primary cAMP assay correlates well with the functional activity seen in the whole blood assay (Fig. 3).
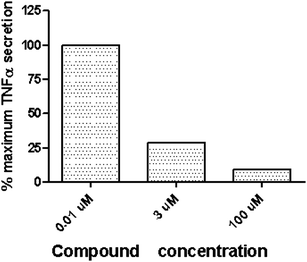 | ||
| Fig. 3 Inhibition of TNFα secretion, ex vivo whole blood LPS challenge for compound 12. Data based on one donor. Normalized to maximum TNFα secreted. | ||
The pharmacological selectivity of 12 was demonstrated through a broad ligand screening panel consisting of 67 targets, showing <40% inhibition at 10 μM at all of these targets. These data coupled with the adequate pharmacokinetic profile of 12 allowed us to select this compound for subsequent in vivo studies. During these studies, treatment dependent changes in heart rate and blood pressure were observed for 12 as well as for another systemically available non-bile acid TGR5 agonist from a structurally distinct chemotype. These data suggest that mechanism-based cardiovascular effects may be associated with systemic TGR5 agonism. The detailed results of this study are beyond the scope of this report and will be disclosed in a future publication.
Conclusions
In conclusion, a potent and orally available triazole-based TGR5 agonist 12 was identified starting from the literature compound 4. 12 demonstrated a robust dog in vivo pharmacokinetic profile that was suitable to allow for further characterization of the TGR5 mechanism, and revealed a safety risk associated with systemic activation of TGR5.Notes and references
- A. Tiwari and P. Maiti, Drug Discovery Today, 2009, 14, 523–530 CrossRef CAS.
- M. Zhong, Curr. Trends Med. Chem., 2010, 10, 386–396 Search PubMed.
- T. W. Pols, L. G. Noriega, M. Nomura, J. Auwerx and K. Schoonjans, J. Hepatol., 2011, 54, 1263–1272 Search PubMed.
- Y. Xu, Annu. Rep. Med. Chem., 2011, 46, 69–87 Search PubMed.
- S. Katsuma, A. Hirasawa and G. Tsujimoto, Biochem. Biophys. Res. Commun., 2005, 329, 386–390 CrossRef CAS.
- M. Watanabe, S. M. Houten, C. Mataki, M. A. Christoffolete, B. W. Kim, H. Sato, N. Messaddeq, J. W. Harney, O. Ezaki, T. Kodama, K. Schoonjans, A. C. Bianco and J. Auwerx, Nature, 2006, 439, 484–489 CrossRef CAS.
- T. W. Pols, M. Nomura, T. Harach, G. Lo Sasso, M. H. Oosterveer, C. Thomas, G. Rizzo, A. Gioiello, L. Adorini, R. Pellicciari, J. Auwerx and K. Schoonjans, Cell Metab., 2011, 14, 747–757 CrossRef CAS.
- R. Pellicciari, A. Gioiello, A. Macchiarulo, C. Thomas, E. Rosatelli, B. Natalini, R. Sardella, M. Pruzanski, A. Roda, E. Pastorini, K. Schoonjans and J. Auwerx, J. Med. Chem., 2009, 52, 7958–7961 CrossRef CAS.
- C. Thomas, A. Gioiello, L. Noriega, A. Strehle, J. Oury, G. Rizzo, A. Macchiarulo, H. Yamamoto, C. Mataki, M. Pruzanski, R. Pellicciari, J. Auwerx and K. Schoonjans, Cell Metab., 2009, 10, 167–177 CrossRef CAS.
- T. Li, S. R. Holmstrom, S. Kir, M. Umetani, D. R. Schmidt, S. A. Kliewer and D. J. Mangelsdorf, Mol. Endocrinol., 2011, 25, 1066–1071 Search PubMed.
- M. R. Herbert, D. L. Siegel, L. Staszewski, C. Cayanan, U. Banerjee, S. Dhamija, J. Anderson, A. Fan, L. Wang, P. Rix, A. K. Shiau, T. S. Rao, S. A. Noble, R. A. Heyman, E. Bischoff, M. Guha, A. Kabakibi and A. B. Pinkerton, Bioorg. Med. Chem. Lett., 2010, 20, 5718–5721 Search PubMed.
- K. A. Evans, B. W. Budzik, S. A. Ross, D. D. Wisnoski, J. Jin, R. A. Rivero, M. Vimal, G. R. Szewczyk, C. Jayawickreme, D. L. Moncol, T. J. Rimele, S. L. Armour, S. P. Weaver, R. J. Griffin, S. M. Tadepalli, M. R. Jeune, T. W. Shearer, Z. B. Chen, L. Chen, D. L. Anderson, J. D. Becherer, M. De Los Frailes and F. J. Colilla, J. Med. Chem., 2009, 52, 7962–7965 Search PubMed.
- B. W. Budzik, K. A. Evans, D. D. Wisnoski, J. Jin, R. A. Rivero, G. R. Szewczyk, C. Jayawickreme, D. L. Moncol and H. Yu, Bioorg. Med. Chem. Lett., 2010, 20, 1363–1367 Search PubMed.
- F. Lombardo, M. Y. Shalaeva, K. A. Tupper and F. Gao, J. Med. Chem., 2001, 44, 2490–2497 CrossRef CAS.
- D. F. Veber, S. R. Johnson, H. Y. Cheng, B. R. Smith, K. W. Ward and K. D. Kopple, J. Med. Chem., 2002, 45, 2615–2623 CrossRef CAS.
- Detailed in vitro pharmacology works around TGR5 will be reported elsewhere.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS.
- A. K. Feldman, B. Colasson and V. V. Fokin, Org. Lett., 2004, 6, 3897–3899 CrossRef CAS.
- S. Bolvig, J. Andersen and X. Liang, Synlett, 2005, 2941–2947 CAS.
- L. H. Jones, N. W. Summerhill, N. A. Swain and J. E. Mills, Med. Chem. Commun., 2010, 1, 309–318 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures for synthesis of compounds 4, 10–18, a dataset of compounds 2–9 used for a plot in Fig. 2, and protocols for cAMP assays, human whole blood assay, and in vivo dog PK studies. See DOI: 10.1039/c2md20174g |
| This journal is © The Royal Society of Chemistry 2013 |