DOI:
10.1039/C2MD20172K
(Review Article)
Med. Chem. Commun., 2013,
4, 27-33
Amino-derivatives of the sesquiterpene lactone class of natural products as prodrugs†
Received
29th June 2012
, Accepted 1st August 2012
First published on 16th August 2012
Abstract
The sesquiterpene lactone class of natural products displays a diverse array of biological activities due to the presence of the α-methylene-γ-lactone motif. However, clinical translation of this class has been hampered by poor aqueous solubility and non-selective binding as a Michael acceptor at undesired targets. A prodrug approach has been developed to overcome these problems in which an amine is added into the α-methylene-γ-lactone to mask this group from nucleophiles and increase solubility. The medicinal chemistry of amino-derivatives of the sesquiterpene lactones is described, beginning with synthetic development, moving into pharmacological applications, and finishing with clinical translation.
Introduction to amino-derivatives of enones
The Michael addition of a primary or secondary amine into an α,β-unsaturated enone is well known in synthetic chemistry yet represents an emerging strategy for medicinal chemistry. These amino-adducts are potentially prodrugs of biologically active molecules, and a thoroughly studied case is the sesquiterpene lactone class of natural products in which each member displays an α-methylene-γ-lactone (Fig. 1). Sesquiterpene lactones exhibit a variety of biological activities, including antitumor, anti-microbial, anti-inflammatory, anti-ulcer and anti-viral activities.1,2 The mechanism of action of these compounds is commonly due to the nucleophilic addition of a biological thiol to the α-methylene-γ-lactone motif.1–3 However, the sesquiterpene lactones typically have poor COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater solubility, and the α-methylene-γ-lactone can exhibit non-selective binding as a Michael acceptor with undesired targets.2 Therefore, an amino-prodrug strategy has been developed in which the reactive α,β-unsaturated enone is masked; additionally, it can enhance aqueous solubility, improve the pharmacokinetic profile, and maintain or even augment the biological activity of the parent molecule. The last characteristic has been hypothesized to occur by elimination of the amine, potentially through bioactivation, to regenerate the α,β-unsaturated enone at the site of action. This prodrug approach has enabled the first successful clinical translation of a member of the sesquiterpene lactones. In this review, a concise description of the medicinal chemistry of amino-derivatives of the sesquiterpene lactones is presented, beginning from its early development in synthesis, moving into its applications in medicinal chemistry, and finishing with its translation into a clinical candidate.
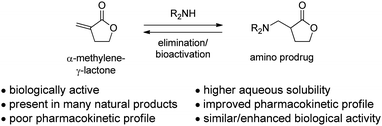 |
| | Fig. 1 Conversion of α-methylene-γ-lactones into amino-derivatives. | |
Originally, the conversion of an α-methylene-γ-lactone into its amino-derivative was used as a method to protect the α,β-unsaturation from a subsequent hydrogenation. In 1968, the sesquiterpene lactone dehydrocostus lactone (1) was transformed into its dimethylamino-adduct to enable selective reduction of two other alkenes (Fig. 2).4N-Alkylation and subsequent pyrolysis reformed the conjugated α-methylene-γ-lactone. Although biological evaluation of the novel derivatives was not conducted, these studies were the first successful application of this strategy to the sesquiterpene lactones.
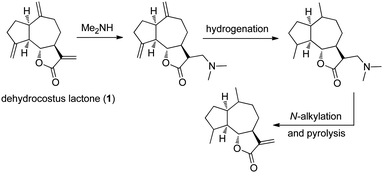 |
| | Fig. 2 Synthesis of amino-derivative of dehydrocostus lactone (1) as a protection strategy for the α,β-unsaturated enone. | |
Helenalin derivatives
Amino-derivatives of the sesquiterpene lactone, COMPOUND LINKS
Read more about this on ChemSpider
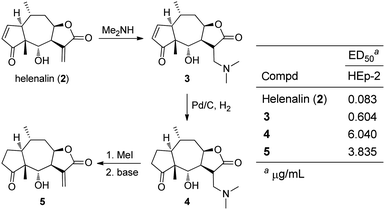
Download mol file of compoundhelenalin (2) were prepared to understand structure–activity relationships of the two α,β-unsaturated carbonyl groups as well as for the Michael-type secondary amine adducts (Fig. 3).5–7 Accordingly, the dimethylamino-analogue 3 was synthesized, and subsequent reduction removed the remaining endo-cyclic α,β-unsaturation.5 The β-amino substituent of 4 was expelled to re-form the α-methylene-γ-lactone of 5 using a mild alkylation/elimination sequence. Biological evaluation of compounds 2–5 using an antiproliferative assay with HEp-2 (human epidermoid carcinoma of the larynx) cells was conducted. The amino-derivative of helenalin 3 (ED50 = 0.604 μg mL−1) displayed an 8-fold loss of activity compared to the parent sesquiterpene lactone2 (ED50 = 0.083 μg mL−1). In a similar fashion, the reduced amino-derivative 4 showed a loss of activity compared to the 2,3-dihydrohelanalin 5, but only a two-fold difference. A series of secondary amino-derivatives of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhelenalin (2) were also prepared using this synthetic strategy, and generally, most amino-adducts displayed a 5–10 fold loss of activity. Analysis of these data led the authors to hypothesize that regeneration of α-methylene-γ-lactone does not occur in cell culture.5 Follow-up in vivo studies on 2 and 3 were performed using male DBA/2 mice with P-388 lymphocytic leukemia, and average survival time was nearly identical for both test agents at 13.4 and 13.0 days, respectively.7 These in vivo data suggest that pharmacokinetic properties may have been enhanced in amino-adduct 3 to compensate for the decrease in antiproliferative potency compared to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhelenalin (2).
The mechanism of the sesquiterpene lactones, such as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhelenalin, is typically due to addition of biological thiols to the α-methylene-γ-lactone motif (Fig. 4).8 Accordingly, complete reduction of both of the α,β-unsaturated groups of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhelenalin provides an inactive analogue (the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclopentenone can also serve as a reactive enone).5 The mechanism of action of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhelenalin (2) is the inhibition of the NF-κB signalling pathway and the induction of reactive oxygen species (ROS),9 likely by COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundglutathione binding.8
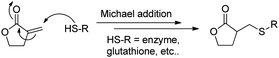 |
| | Fig. 4 Mechanism of action of α-methylene-γ-lactones by thiol trapping. | |
Ambrosin derivatives
In 1995, amino-derivatives of the sesquiterpene lactone, ambrosin (6), were synthesized by Cushman and co-workers, because clinical development of the natural product as an anticancer agent was limited by poor aqueous solubility (Fig. 5).10 The amines were immediately converted into their respective hydrochloride salts and evaluated in 51 different cancer cell lines. The pyrrolidine derivative 7 and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpiperidine analogue 8 manifested antiproliferative activity most similar to the parent compound 6 across the majority of the cell lines. For example, biological activities for 6–8 in lung cancer (NCI-H522), melanoma (LOX-IMVI), and breast cancer (MCF7) cells are nearly identical. Additionally, there was a substantial increase in the aqueous solubilities of the amine hydrochlorides 7 and 8, but the exact levels of aqueous solubility were not quantified.
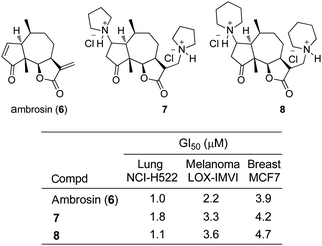 |
| | Fig. 5 Structures of ambrosin (6) and its amino-derivatives 7–8 along with antiproliferative assay data. | |
Cushman posited that conversion of the amino-derivatives back into the parent compound could be accomplished through metabolic N-oxidation of the tertiary amine followed by a Cope elimination of the corresponding N-oxide to give ambrosin 6 (Fig. 6).10 This mechanistic hypothesis, coupled with the parity of the biological data, indicated that the amino-derivatives served as water-soluble prodrugs of ambrosin 6. Indeed, a prodrug strategy involving the one-step preparation of amino-adducts with enhanced COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater solubility, yet without altered biological activity, was a powerful notion. Similar to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhelenalin2, ambrosin 6 inhibits NF-κB signalling,11 and the loss of both α,β-unsaturated groups of ambrosin 6 also provides inactive derivatives.10 Helenalin 2 and ambrosin 6 inhibit inducible nitric oxide synthase (iNOS)-dependent nitric oxide (NO) synthesis by decreasing nitrite accumulation in lipopolysaccharide (LPS)-activated RAW 264.7 macrophages.11
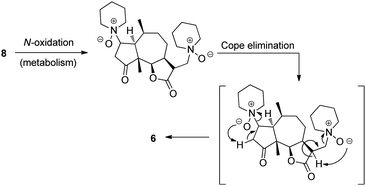 |
| | Fig. 6 Conversion of the ambrosin prodrug8 into ambrosin 6. | |
Read more about this on ChemSpider
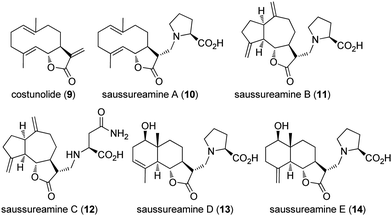
Download mol file of compoundsaussureamine A (10) (Fig. 7). COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundSaussureamine B (11) and C (12) are proline- and asparagine-adducts of the dehydrocostus lactone1, respectively. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundSaussureamine D (13) and E (14) are proline-derivatives of the natural products, santamarine and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundreynosin.13 Because all of the sesquiterpene lactones1, 9, and 10–14 are readily isolated from the roots of Saussurea lappa, side-by-side biological evaluations of these agents have been conducted to compare gastro-protective effects12,13 and inhibition of inflammation (Table 1).14 Costunolide 9 and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsaussureamine A (10) produce similar levels of inhibition of nitric oxide production in macrophages. In contrast, 9 is a more potent inhibitor than 10 for decreasing HCl/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundEtOH induced gastric lesions, yet 10 is a more potent inhibitor of water-immersion induced lesions in mice than 9. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundSaussureamine B (11) displays identical biological activity to dehydrocostus lactone1 in all three assays; however, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsaussureamine C (12) manifests the least biological activity in nearly all three assays. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundSaussureamine B (11) is an improved candidate over 1, because it is significantly more soluble in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater.13 Additional mechanism of action studies on 1, 9, 10 and 11 confirmed that all of these molecules exhibited anti-inflammation activity by inhibiting iNOS enzyme activity, iNOS and HSP 72 proteins, and NF-κB activation.14 In contrast, it is known that reduction of the exo-methylene of the α-methylene-γ-lactone of costunolide 9 results in an inactive derivative.15
Table 1 Biological activities of 1, 9, and 10–14
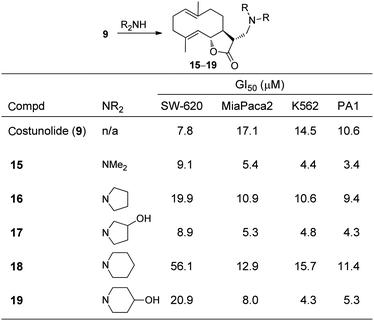
Sixteen amino-adducts of costunolide 9 were synthesized and tested in eight different cancer cell lines.16 Typically, the amine-analogues derived from COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddimethylamine, pyrrolidines, or piperidines displayed significant antiproliferative activity similar to the parent 9; however, all of the compounds prepared from piperazines were inactive or weakly active. A subset of data from the active derivatives 15–19 is illustrated in Fig. 8. The COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddimethylamine15, the 3-hydroxypyrrolidine 17, and the 4-hydroxypiperidine 19 exhibited enhanced antiproliferative activities in MiaPaca2 (pancreatic cancer), K562 (leukemia), and PA1 (ovarian cancer) cells compared to 9. In contrast, piperidine18 displays a dramatic loss of activity compared to 9 in SW-620 (colon cancer) cells. These data demonstrated that understanding structure–activity relationships across amine-derivatives of a sesquiterpene lactone is fundamental to identifying candidate compounds for different cancers. All of the analogues were additionally screened against normal mouse fibroblast (NIH3T3) cells to establish a safety profile, and typically, the amine-adducts demonstrated less cytotoxicity than 9. Indeed, it is known from studies by Blagg and co-workers that adding a basic nitrogen atom into the structure of a cytotoxic agent can enhance selectivity for malignant cell lines.17
Alantolactone derivatives
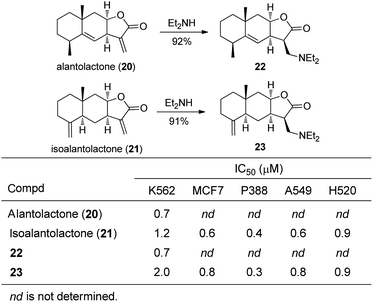
Three amino-adducts of the sesquiterpene lactone alantolactone (20) and twelve derivatives of isoalantolactone (21) were synthesized and subjected to evaluation in antiproliferative assays with K562 cells (Fig. 9).18 Like other studies, a broad range of potencies were observed, but most were similar to the parent compounds. The diethylamine-derivatives of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundalantolactone (22) and isoalantolactone (23) were selected as candidates for additional screening for effects on cell cycle distribution, and the latter agent was examined against the Paterson panel of tumour cell lines (Fig. 9). Both of the amines22 and 23 demonstrated the capacity to produce similar distributions of cell cycle changes in K562 cells compared to 20 and 21. To account for the similar biological activities, the authors hypothesized that the Michael addition of the amine must be reversible to allow subsequent alkylation of nucleophilic biomolecules. As for the mechanism of action of the natural product, alantolactone 20 inhibits iNOS-dependent NO synthesis by decreasing nitrite accumulation but is nearly inactive in the NF-κB signalling pathway.11
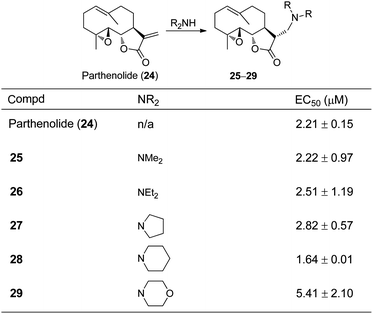
Parthenolide derivatives
The sesquiterpene lactone parthenolide represents the most well studied case for the development of an amino-prodrug, because the promising biological activity of the natural product has driven substantial efforts for its clinical translation as an anticancer agent.19 Using inspiration from Cushman et al.10 and Yoshikawa et al.,12 the first report of amino-adducts of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundparthenolide (24) described the preparation of its prodrugs for hepatitis C virus (HCV) infection (Fig. 10).20 Seven parthenolide derivatives were prepared from secondary amines and evaluated for anti-HCV effects, and typically, the amines demonstrated activity similar to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundparthenolide24.20 A subset of the reported data is listed for analogues 25–29 in Fig. 10. Accordingly, the anti-HCV effects for 24 (EC50 = 2.21 μM) are nearly identical to analogues 25–27. Additionally, lysates from cells treated with amino-adducts and subsequently analyzed by LC/MS/MS revealed the presence of the parent compound, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundparthenolide24, but no residual amino-derivatives.20 These data correlate with the prior work of Matsuda in that the amino-analogues display the same mechanism of action as the parent compound (with an α-methylene-γ-lactone) in cellular assays.14
Following a report that COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundparthenolide selectively targets and initiates apoptosis in leukemia stem cells,19 Crooks and co-workers synthesized a series of amino-derivatives of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundparthenolide from primary and secondary amines.21,22 The dimethylamino parthenolide 25 (also referred to as DMAPT or LC-1) was shown to have excellent oral bioavailability, superior aqueous solubility, and also the superb anti-leukemic activity of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundparthenolide24.23,24 Structure–activity investigations in 60 human cancer cell lines (NCI-60) of analogues of 24 from the fourteen primary amines and three secondary amines identified compound 30 with antiproliferative activity that is superior to 24 (Fig. 11).21 Specifically, analogue 30 manifests GI50 < 0.01 μM in CCRF-CEM (lymphoblastic leukemia) cells compared to GI50 = 8.38 μM for COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundparthenolide24. Most other analogues displayed similar potencies to 24. Subsequent studies by Crooks et al. detailed anti-leukemic activity in AML (acute myelogenous leukemia) cells for thirty-two aminoparthenolides.22 A portion of these data are listed for analogues 25–29 for comparisons (Table 2). A larger range of cell killing was demonstrated across all of these analogues, likely due to the broad range of structures of the amines examined. DMAPT 25 was determined to be the candidate compound for subsequent advancement to pre-clinical studies and clinical studies.23–25 The anti-leukemic activity of parthenolide 24 manifests from its ability to inhibit NF-κB as well as initiate oxidative stress by increasing ROS.19 DMAPT 25 also inhibits NF-κB and causes oxidative stress.23,26 Analogous to prior structure–activity studies for other sesquiterpene lactones, reduction of the α,β-unsaturation of the α-methylene-γ-lactone of parthenolide 24 provides an inactive derivative.27
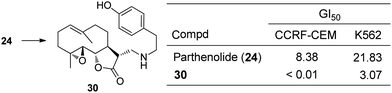 |
| | Fig. 11 Antiproliferative activity of parthenolide 24 and aminoparthenolide 30. | |
Table 2 Anti-leukemic activity of parthenolide 24 and analogues 25–29
Preliminary stability studies determined that less than 3% of DMAPT 25 degraded to parthenolide 24 over 24 h.22 Only a small amount of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundparthenolide was found in blood plasma of rats eight hours after dosing with DMAPT 25. Oral bioavailability for DMAPT 25 was 70% in rats,22 mice, and canine models.23 On the other hand, parthenolide 24 has a poor pharmacokinetic profile, and phase I clinical studies determined that 24 was undetectable in patients' plasma samples after oral dosing.28 Concentration maximum (Cmax) and half-life (T1/2) for oral dosing of DMAPT 25 (100 mg kg−1) was 25 μM and 0.63 hours in mice and 61 μM and 1.9 hours in canines.23 Parthenolide 24 displays Cmax = 200 nM in mice after a dose of 40 mg kg−1, which is the highest dose allowed due to its poor solubility. Also, parthenolide 24 is highly protein-bound, through its action as a Michael acceptor, after incubation in human plasma and human serum albumin.29 DMAPT 25 has an excellent solubility profile and is over 1000-fold more soluble in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater relative to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundparthenolide24.23,26
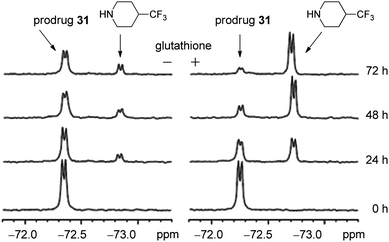
Fluorinated aminoparthenolides, derived from pyrrolidines and piperidines, were synthesized by Colby and co-workers for mechanistic analysis by 19F NMR.30 Six fluorinated piperidine analogues and three fluorinated pyrrolidine derivatives were made and compared to 24, 27, and 28 in an antiproliferative assay in HL-60 cells; 4-trifluoromethylpiperidine 31 was determined to have biological activity most similar to 24 (Fig. 12). Using 19F NMR in a deuterium-free environment, it was found that the fluorinated amine was released from the aminoparthenolide 31 in the presence of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundglutathione, yet this effect was minimal in its absence (Fig. 13). These data support that the conversion of an aminoparthenolide to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundparthenolide is promoted in the presence of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundglutathione, which is normally more prevalent in malignant cells, and also that an aminoparthenolide can serve as a prodrug of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundparthenolide. 19F NMR observes interesting fluorinated drug candidates and their fates under physiological conditions with almost zero background interference and minimal efforts in sample preparation.30 Using deuterium-free environments during concentration analysis by NMR is preferable when replicating processes that occur in aqueous systems.30,31 Moreover, it is particularly advantageous to apply this method when complex systems are present or when multiple metabolic events/biochemical pathways can take place. In this example, 19F NMR clearly showed the dissociation of the fluorinated amine from prodrug31 (as expected) with minimal interference.30
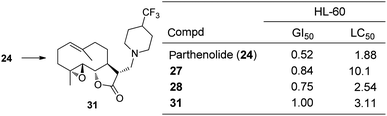 |
| | Fig. 12 Fluorinated aminoparthenolide 31. | |
Conclusions
The sesquiterpene lactone class of natural products displays a diverse array of biological activities due to the presence of the α-methylene-γ-lactone. However, clinical translation of this class has been hampered by poor aqueous solubility and non-selective binding as a Michael acceptor at undesired targets. A prodrug approach has been developed to overcome these problems in which an amine is added into the α-methylene-γ-lactone to mask this Michael acceptor from undesired nucleophiles and increase COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater solubility. Similar biological activities and mechanisms of action between the amino-adducts and the parent sesquiterpene lactones have been continuously demonstrated across COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhelenalin, ambrosin, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcostunolide, saussureamines, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundalantolactone, and COMPOUND LINKS
Read more about this on ChemSpider
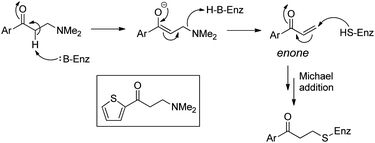
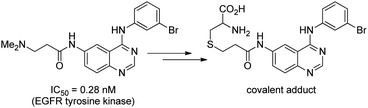
Download mol file of compoundparthenolide. Clinical translation of the aminoparthenolide, DMAPT, is of the utmost significance in medicinal chemistry, especially due to the strong aversion to covalent mechanism of action.3 This prodrug strategy has additional implications beyond the sesquiterpene lactones and presents a larger opportunity to modify lead structures that contain reactive enones. Accordingly, inhibitors of the sortase enzyme have been discovered from aryl β-amino-ethyl ketones (Fig. 14).32Mass spectrometry and X-ray crystallographic studies revealed a thienylpropanone covalently tethered to the β-position of the inhibitor in the active site of the enzyme. More recently in 2012, 3-aminopropanamides were found to be inhibitors of epidermal growth factor receptor (EGFR), and HPLC-HRMS of cell lysates treated with these agents revealed accumulation of the corresponding acrylamide and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundacrylamide–cysteine conjugates (Fig. 15).33 The identification of dimethylamino-adducts as lead compounds from these two studies can be anticipated based on the successes of dimethylamino-derivatives of the sesquiterpene lactones. Overall, this amino-prodrug approach provides, in one synthetic step, an opportunity to enhance COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater solubility, improve the pharmacokinetic profile, and maintain (or enhance) the biological activity of an enone-containing lead compound; however, a series of amino-analogues must be prepared to ensure identification of the best candidate. This approach is a substantial innovation, especially as new biologically active molecules with key α,β-unsaturated enones continue to be identified.34,35 Future efforts to expand the application of this type of amino-prodrug strategy will likely be directed toward establishing that the amino-derivatives display fewer effects from non-selective binding than the parent enones. Also, validating the mechanism of biological activation for the amino-derivatives in a living system would be a substantial advancement.
Notes and references
- R. R. A. Kitson, A. Millemaggi and R. J. K. Taylor, Angew. Chem., Int. Ed., 2009, 48, 9426–9451 CrossRef CAS.
- A. Ghantous, H. Gali-Muhtasib, H. Vuorela, N. A. Saliba and N. Darwiche, Drug Discovery Today, 2010, 15, 668–678 CrossRef CAS.
- C. Avonto, O. Taglialatela-Scafati, F. Pollastro, A. Minassi, V. Di Marzo, L. De Petrocellis and G. Appendino, Angew. Chem., Int. Ed., 2011, 50, 467–471 CrossRef CAS.
- N. R. Unde, S. V. Hiremath, G. H. Kulkarni and G. R. Kelkar, Tetrahedron Lett., 1968, 9, 4861–4862 CrossRef.
- K.-H. Lee, H. Furukawa and E.-S. Huang, J. Med. Chem., 1972, 15, 609–611 CrossRef CAS.
- K.-H. Lee, S.-H. Kim, H. Furukawa, C. Piantadosi and E.-S. Huang, J. Med. Chem., 1975, 18, 59–63 CrossRef CAS.
- K.-H. Lee, T. Ibuka, E.-C. Mar and I. H. Hall, J. Med. Chem., 1978, 21, 698–701 CrossRef CAS.
- T. J. Schmidt, Bioorg. Med. Chem., 1997, 5, 645–653 CrossRef CAS.
- R. Hoffmann, K. von Schwarzenberg, N. López-Antón, A. Rudy, G. Wanner, V. M. Dirsch and A. M. Vollmar, Biochem. Pharmacol., 2011, 82, 453–463 CrossRef CAS.
- E. Hejchman, R. D. Haugwitz and M. Cushman, J. Med. Chem., 1995, 38, 3407–3410 CrossRef CAS.
- V. M. Dirsch, H. Stuppner, E. P. Ellmerer-Müller and A. M. Vollmar, Bioorg. Med. Chem., 2000, 8, 2747–2753 CrossRef CAS.
- M. Yoshikawa, S. Hatakeyama, Y. Inoue and J. Yamahara, Chem. Pharm. Bull., 1993, 41, 214–216 CrossRef CAS.
- H. Matsuda, T. Kageura, Y. Inoue, T. Morikawa and M. Yoshikawa, Tetrahedron, 2000, 56, 7763–7777 CrossRef CAS.
- H. Matsuda, I. Toguchida, K. Ninomiya, T. Kageura, T. Morikawa and M. Yoshikawa, Bioorg. Med. Chem., 2003, 11, 709–715 CrossRef CAS.
- C.-M. Sun, W. Syu, Jr, M.-J. Don, J.-J. Lu and G.-H. Lee, J. Nat. Prod., 2003, 66, 1175–1180 CrossRef CAS.
- S. K. Srivastava, A. Abraham, B. Bhat, M. Jaggi, A. T. Singh, V. K. Sanna, G. Singh, S. K. Agarwal, R. Mukherjee and A. C. Burman, Bioorg. Med. Chem. Lett., 2006, 16, 4195–4199 CrossRef CAS.
- M. Duvvuri, S. Konkar, K. H. Hong, B. S. J. Blagg and J. P. Krise, ACS Chem. Biol., 2006, 1, 309–315 CrossRef CAS.
- N. J. Lawrence, A. T. McGown, J. Nduka, J. A. Hadfield and R. G. Pritchard, Bioorg. Med. Chem. Lett., 2001, 11, 429–431 CrossRef CAS.
- M. L. Guzman, R. M. Rossi, L. Karnischky, X. Li, D. R. Peterson, D. S. Howard and C. T. Jordan, Blood, 2005, 105, 4163–4169 CrossRef CAS.
- D.-R. Hwang, Y.-S. Wu, C.-W. Chang, T.-W. Lien, W.-C. Chen, U.-K. Tan, J. T. A. Hsu and H.-P. Hsieh, Bioorg. Med. Chem., 2006, 14, 83–91 CrossRef CAS.
- S. Nasim and P. A. Crooks, Bioorg. Med. Chem. Lett., 2008, 18, 3870–3873 CrossRef CAS.
- S. Neelakantan, S. Nasim, M. L. Guzman, C. T. Jordan and P. A. Crooks, Bioorg. Med. Chem. Lett., 2009, 19, 4346–4349 CrossRef CAS.
- M. L. Guzman, R. M. Rossi, S. Neelakantan, X. Li, C. A. Corbett, D. C. Hassane, M. W. Becker, J. M. Bennett, E. Sullivan, J. L. Lachowicz, A. Vaughan, C. J. Sweeney, W. Matthews, M. Carroll, J. L. Liesveld, P. A. Crooks and C. T. Jordan, Blood, 2007, 110, 4427–4435 CrossRef CAS.
- D. C. Hassane, S. Sen, M. Minhajuddin, R. M. Rossi, C. A. Corbett, M. Balys, L. Wei, P. A. Crooks, M. L. Guzman and C. T. Jordan, Blood, 2010, 116, 5983–5990 CrossRef CAS.
- S. Hewamana, T. T. Lin, C. Jenkins, A. K. Burnett, C. T. Jordan, C. Fegan, P. Brennan, C. Rowntree and C. Pepper, Clin. Cancer Res., 2008, 14, 8102–8111 CrossRef CAS.
- R. Shanmugam, P. Kusumanchi, L. Cheng, P. Crooks, S. Neelakantan, W. Matthews, H. Nakshatri and C. J. Sweeney, Prostate, 2010, 70, 1074–1086 CrossRef CAS.
- B. H. B. Kwok, B. Koh, M. I. Ndubuisi, M. Elofsson and C. M. Crews, Chem. Biol., 2001, 8, 759–766 CrossRef CAS.
- E. A. Curry III, D. J. Murry, C. Yoder, K. Fife, V. Armstrong, H. Nakshatri, M. O'Connell and C. J. Sweeney, Invest. New Drugs, 2004, 22, 299–305 CrossRef.
- S. Wagner, F. Kratz and I. Merfort, Planta Med., 2004, 70, 227–233 CrossRef CAS.
- J. R. Woods, H. Mo, A. A. Bieberich, T. Alavanja and D. A. Colby, J. Med. Chem., 2011, 54, 7934–7941 CrossRef CAS.
- H. Mo, K. M. Balko and D. A. Colby, Bioorg. Med. Chem. Lett., 2010, 20, 6712–6715 CrossRef CAS.
- A. W. Maresso, R. Wu, J. W. Kern, R. Zhang, D. Janik, D. M. Missiakas, M.-E. Duban, A. Joachimiak and O. Schneewind, J. Biol. Chem., 2007, 282, 23129–23139 CrossRef CAS.
- C. Carmi, E. Galvani, F. Vacondio, S. Rivara, A. Lodola, S. Russo, S. Aiello, F. Bordi, G. Costantino, A. Cavazzoni, R. R. Alfieri, A. Ardizzoni, P. G. Petronini and M. Mor, J. Med. Chem., 2012, 55, 2251–2264 CrossRef CAS.
- J. K. Hexum, R. Tello-Aburto, N. B. Struntz, A. M. Harned and D. A. Harki, ACS Med. Chem. Lett., 2012, 3, 459–464 CrossRef CAS.
- M. V. Riofski, J. P. John, M. M. Zheng, J. Kirshner and D. A. Colby, J. Org. Chem., 2011, 76, 3676–3683 CrossRef CAS.
Footnote |
| † This article is part of a MedChemComm ‘New Talents’ issue highlighting the work of outstanding rising scientists in medicinal chemistry research. |
|
| This journal is © The Royal Society of Chemistry 2013 |
Click here to see how this site uses Cookies. View our privacy policy here.