 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dissecting the salt dependence of the Tus–Ter protein–DNA complexes by high-throughput differential scanning fluorimetry of a GFP-tagged Tus†
Morgane J. J. Moreauab and Patrick M. Schaeffer‡*ab
aCentre for Biodiscovery and Molecular Development of Therapeutics, James Cook University, Douglas, QLD 4811, Australia
bComparative Genomics Centre, James Cook University, Douglas, QLD 4811, Australia
First published on 1st October 2013
Abstract
The analysis of the salt dependence of protein–DNA complexes provides useful information about the non-specific electrostatic and sequence-specific parameters driving complex formation and stability. The differential scanning fluorimetry of GFP-tagged protein (DSF-GTP) assay has been geared with an automatic Tm peak recognition system and was applied for the high-throughput (HT) determination of salt-induced effects on the GFP-tagged DNA replication protein Tus in complex with various Ter and Ter-lock sequences. The system was designed to generate two-dimensional heat map profiles of Tus-GFP protein stability allowing for a comparative study of the effect of eight increasing salt concentrations on ten different Ter DNA species at once. The data obtained with the new HT DSF-GTP allowed precise dissection of the non-specific electrostatic and sequence-specific parameters driving Tus–Ter and Tus–Ter-lock complex formation and stability. The major factor increasing the thermal resistance of Tus–Ter-lock complexes in high-salt is the formation of the TT-lock, e.g. a 10-fold higher Kspe was obtained for Tus-GFP:Ter-lockB than for Tus-GFP:TerB. It is anticipated that the system can be easily adapted for the study of other protein–DNA complexes.
1. Introduction
The affinity and stability of protein–DNA complexes are characterized by the binding constant and Gibbs energy of binding, but these parameters are not sufficient to dissect the nature of the physical forces involved in the interaction – i.e. non-specific electrostatic contacts vs. sequence-specific contacts. An experimental approach that analyzed the effects of salt on the stability of protein–DNA complexes was developed nearly four decades ago by Record et al.1,2 It assumes that the release of counter-ions associated with free DNA is a major factor in protein–DNA complex formation.1–6 The application of the concept of counter-ion condensation (CC) allows determination of the electrostatic component in a protein–DNA complex. The analysis of the salt dependence of the binding constant can also be used to determine the non-electrostatic component of the interaction, which is sequence-specific. The relative magnitude and importance of each component modulates the functional specificity and affinity of a protein for various binding sites.6 As a rule, the salt resistance of a protein–DNA complex indicates a dominant contribution of sequence-specific interactions.Tus is a relatively well characterized protein that binds specifically to 21 bp DNA sequences, called Ter, scattered around the circular chromosome of E. coli (Fig. 1A) to coordinate the termination of DNA replication opposite to oriC.7–22 The Tus–Ter complex can only arrest a replisome approaching towards its non-permissive face through the formation of a Tus–Ter-lock (TT-lock),18 whose action is to tighten the complex, avoiding dissociation of Tus. When the replisome approaches the permissive face of the complex, Tus rapidly dissociates from Ter. The TT-lock is formed when the C·G(6) base pair is broken during DNA unwinding at the non-permissive face of the complex. The unpaired C(6) can then bind into a cytosine-binding pocket at the surface of Tus.18 The ‘polarity’ of the Tus–Ter complex has also been proposed to be the result of a specific interaction occurring between Tus and the DnaB helicase at the forefront of the replisome.7,19 All together, 14 Ter sites have been identified.11 While the role of the innermost strong Tus–Ter complexes (Tus–TerA–D, Fig. 1A) in DNA replication termination is clear, the presence and organization of the outer Ter sites is still enigmatic.11,16 The characterization of the outer Ter sites is therefore of importance to understand their function and role in the wide replication fork trap of E. coli (Fig. 1A).
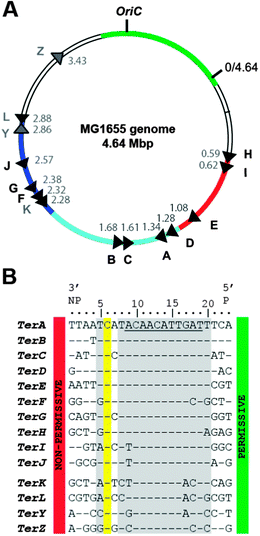 | ||
| Fig. 1 Position, orientation and sequences of Ter sites in E. coli MG1655. (A) Map of the E. coli genome indicating the approximate positions and orientations of the 14 Ter sites.11 The tip of the arrow indicates the non-permissive face of the Tus–Ter complex. The coloured regions on the circle represent chromosome domains: oriC (green); unstructured domains (colourless); the right domain (red); the left domain (dark blue); and the termination domain (light blue).27,28 The numbers inside the circle indicate Ter site positions in Mbp. (B) Sequence similarities of the 14 Ter sites. The conserved C(6) is highlighted in yellow. Sequences are oriented with their non-permissive face (NP) on the left. Nucleotides interacting with Tus are shaded in grey.12 The 11-bp core sequence determined by Coskun-Ari and Hill is underlined.9 | ||
The effect of salt on the association and dissociation kinetics of Tus–TerB was previously examined by surface plasmon resonance (SPR).20 Later, the effect of salt on the dissociation kinetics of the TT-lock variant of TerB (Ter-lockB) was also examined, revealing an increased resistance to salt by this species compared to TerB.18 The kinetic parameters of the primary Ter sites, TerA–J, and their TT-lock variants have recently been characterized by SPR at two different salt concentrations.16 Unfortunately, weak binders could not be characterized under high salt conditions and strong binders under low salt conditions. Notably, TerF was shown to be only marginally more specific than a non-specific oriC DNA fragment, suggesting that TerF forms mainly non-specific electrostatic interactions with Tus, and raising questions about its role in DNA replication termination.16 The study revealed the limitation of using SPR for these studies as the data were complicated by non-specific binding of Tus to the surface of the chip in low salt and extreme bulk shift effect under high salt conditions. Using a newly developed DSF-GTP assay we recently investigated the effect of salt on Tus-GFP:TerB and Tus-GFP:Ter-lockB complexes.17 Nevertheless, the limited and scattered data we and others obtained on the effect of ionic strength on the Tus–Ter complex prompted us to further investigate this important system.
In this study the effect of very low to high ionic strength on the stability of the ten primary Tus–Ter and their Tus–Ter-lock complexes was systematically analyzed using DSF-GTP17 equipped with an automatic Tm peak recognition system. The new automatic Tm peak recognition system is able to automatically generate two-dimensional (2D) heat maps with an accuracy of 96% and greatly accelerates data analysis. Using this system, the effect of increasing concentrations of potassium chloride on the overall stability of these complexes could be determined in high-throughput and revealed the subtle differences in affinity between the different Ter species and their TT-lock variants.
2. Materials and methods
2.1. Protein expression and purification
His6-Tus-GFP was expressed and purified as previously described,10 however proteins were resuspended in SPR250 buffer (50 mM Tris (pH 7.6), 250 mM KCl, 0.1 mM EDTA and 0.2 mM β-mercaptoethanol) and dialysed twice against the same buffer at 4 °C.2.2. Effect of ionic strength on Tus-GFP:Ter complex stability
Tus-GFP was incubated with Ter in the presence of various KCl concentrations and subjected to the melting curve program of a real-time thermal cycler (IQ5 iCycler, Bio-rad). For this, equal volumes of Tus-GFP (7.5 μM), hybridized oligonucleotides (9 μM) and KCl (25.5–1050 mM) were mixed in a qPCR 96-well plate (Bio-Rad) to yield final reaction conditions of 50 mM Tris (pH 7.7), 0.1 mM EDTA, 0.2 mM β-mercaptoethanol supplemented with 8.5–350 mM KCl. Ter DNA was in slight excess (3 μM) compared to Tus-GFP (2.5 μM). The mixture was left 10 min at room temperature to reach equilibrium before starting the melting curve protocol. The melting curve program was run from 35 °C to 75 °C, 0.5 °C per cycle, 30 s dwell time. The Tm values were obtained from the first peak maximum in the melting curves either by graphical analysis17 or using the automatic peak recognition program developed with the free RStudio interface (as described below). Experiments were performed in triplicate.2.3. Automatic Tm peak recognition using RStudio
The following program was adapted from Thermal Shift Assays – Xtalwiki. After a DSF-GTP run, the RFU and −dRFU/dT data were exported to Excel and saved as CSV files. The following script commands RStudio to read the CSV files (italicized characters are to be adapted for each user, run or file name).raw_data<-read.csv(“C:/Documents and settings/path to file/RFUfile.csv”)
grad_data<-read.csv(“C:/Documents and settings/path to file/−dRFUfile.csv”)
The following script commands RStudio to scale plots of RFUs and −dRFU/dT on a single graph and determine Tm at the maximum of the derivative function between 35 °C and 71.5 °C to avoid taking into account the peak corresponding to GFP unfolding. It then generates individual plots for each well in a pdf file.
find.tm<-function(temp=temp, I=I, grad=grad, well=well) {
Igrad<-matrix(1:154,nc=2)
Igrad[,1]=I
Igrad[,2]=grad
scaled_data<-scale(Igrad)
plot(x=temp, y=scaled_data[,1], type=‘p’, col=‘red’, xlab=‘‘’’, ylab=‘‘’’,ylim=c(−5,5))
lines(x=temp, y=scaled_data[,2], type=‘l’, col=‘blue’, lwd=2,xlab=‘‘’’, ylab=‘‘’’)
title(main=well)
tm.s=temp[which.max(Igrad[1:73,2])]
title(sub=sprintf(“Tm=%4.1f”, tm.s, cex.sub=1.2))
return(tm.s)}
pdf(file=“C:/Documents and settings/username/path to file/Thermographs.pdf”, width=30, height=21, pointsize=9)
layout(matrix(data=1:96, nrow=8, ncol=12, byrow=TRUE))
tma<-matrix(nrow=12, ncol=8)
for(i in 2:97) {try(expr=tma[i-1]<-find.tm(temp=raw_data[,1], I=raw_data[,i], grad=grad_data[,i], well=names(raw_data)[i]))}
dev.off()
It has to be noted that in line 2 of the above script (see the number in bold), the matrix scale has to be adapted for the range of temperatures tested in a particular experiment (i.e. ramping speed determines the number of rows in the database) and can be generally calculated as follows: (total number of rows in the dataset − 1) × 2. In the above example, the temperature range was 35 °C to 73.5 °C with 0.5 °C increments. In line 11, the numbers in bold indicate the range of rows corresponding to the temperatures over which the program identifies the highest value on the y-axis as Tm. Since the peak corresponding to the unfolding of GFP starts at around 72 °C and rapidly increases, the maximum temperature used for Tm determination was 71.5 °C to avoid false peak identification.
The following script generates a 2D heat map of the 96-well plate with a color gradient code from red (low Tm) to yellow (high Tm):
pdf(file=“C:/Documents and settings/username/Desktop/2Dheatmap.pdf”, width=6, height=5, paper=“a4”, pointsize=8)
tmaplot<-matrix(nrow=12,ncol=8,data=0)
for(i in 1:8) {tmaplot[,9-i]=tma[,i]}
image(tmaplot)
dev.off()
The correlation between Tm values obtained through the automatic peak recognition system and the visual curve analysis was tested using the Pearson r test in GraphPad Prism.
2.4. Determination of the number of ionic contacts and specific interactions Kspe
The variation in ionic contacts (N) and Kspe was determined for TerB, Ter-lockB, TerF, Ter-lockF, TerJ and Ter-lockJ. Tus-GFP (2.5 μM) was incubated with Ter or Ter-lock DNA at concentrations ranging from 0.6–10 μM in the presence of increasing [KCl] and subjected to the melting curve program of a real-time thermal cycler (IQ5 iCycler, Bio-rad). For this, equal volumes of Tus-GFP (7.5 μM), Ter or Ter-lock (1.8–30 μM) and [KCl] (450 to 1275 mM) were mixed in a qPCR 96-well plate (Bio-Rad) to yield reactions containing 50 mM Tris (pH 7.5), 0.1 mM EDTA, 0.2 mM β-mercaptoethanol supplemented with 150–425 mM [KCl]. The mixture was left 10 min at room temperature to equilibrate before starting the melting curve protocol. The melting curve program was run from 35 °C to 85 °C, 0.5 °C per cycle, 30 s dwell time. The Tm values were obtained from the first peak maximum in the melting curves using the automatic peak recognition program. Experiments were performed in duplicate.The DNA-induced stability (ΔTm) was calculated by subtracting the Tm value obtained for free Tus-GFP from the Tm value obtained for Tus-GFP:Ter complexes at a given KCl concentration. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kobs values were obtained for each [KCl] and Ter or Ter-lock species as previously described17 by plotting the ΔTmvs. log[Ter] and extrapolation of the linear part of the slope (the intersection of the extrapolated slope with the x-axis represents log
Kobs values were obtained for each [KCl] and Ter or Ter-lock species as previously described17 by plotting the ΔTmvs. log[Ter] and extrapolation of the linear part of the slope (the intersection of the extrapolated slope with the x-axis represents log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kobs). The slopes (SKA) were obtained by linear regression of log
Kobs). The slopes (SKA) were obtained by linear regression of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KA (KA = 1/Kobs) vs. log[KCl] according to Record et al.2SKA values were used to determine the number of cations displaced (N) = −SKA/0.88 and Kspe was determined by extrapolation of the SKA at [KCl] = 1 M.
KA (KA = 1/Kobs) vs. log[KCl] according to Record et al.2SKA values were used to determine the number of cations displaced (N) = −SKA/0.88 and Kspe was determined by extrapolation of the SKA at [KCl] = 1 M.
3. Results and discussion
3.1. Automatic determination of Tm values and generation of 2D heat maps
As a first step, it was essential to increase the throughput of the DSF-GTP assay to be able to handle the volume of data generated in this study. A universal, automatic Tm peak recognition program was developed for the RStudio interface (http://www.rstudio.org) that produces a 2D heat map of a 96-well plate directly from raw data. This script provides individual thermoplots of normalized RFU and −dRFU/dT variables and reports the temperature at the maximum value of the derivative as Tm (see Material and methods and Fig. 2A). The representative thermoplots obtained for free and TerC-bound Tus-GFP at increasing [KCl] are shown in Fig. 2A. The Tm values extracted from the thermoplots were then automatically incorporated into 2D-heat maps (Fig. 2B and C).![Automatic determination of Tm for free and DNA-bound Tus-GFP. (A) Examples of thermoplots. RFU signal (red) and its derivative −dRFU/dT (blue) obtained for free or TerC-bound Tus-GFP with increasing [KCl] (8.5–250 mM). (B) 2D heat map of Tm values obtained for Tus-GFP:Ter complexes with increasing [KCl] (8.5–350 mM). Tus-GFP (2.5 μM) and Ter or oriC DNA (3 μM) in SPR buffer supplemented with increasing [KCl]. NA: not available. Tm values range: 40 °C (red) to 71.5 °C (pale yellow). (C) 2D heat map for Tus-GFP:Ter-lock complexes. (D) Pearson's r correlation between averaged Tm values (n = 3) obtained by visual analysis and by automatic Tm peak analysis of DSF-GTP curves (n = 184; data from free Tus-GFP and in complex with Ter, Ter-lock, oriC, oriC-lock). Red error bars: 95% confidence interval.](/image/article/2013/MB/c3mb70426b/c3mb70426b-f2.gif) | ||
| Fig. 2 Automatic determination of Tm for free and DNA-bound Tus-GFP. (A) Examples of thermoplots. RFU signal (red) and its derivative −dRFU/dT (blue) obtained for free or TerC-bound Tus-GFP with increasing [KCl] (8.5–250 mM). (B) 2D heat map of Tm values obtained for Tus-GFP:Ter complexes with increasing [KCl] (8.5–350 mM). Tus-GFP (2.5 μM) and Ter or oriC DNA (3 μM) in SPR buffer supplemented with increasing [KCl]. NA: not available. Tm values range: 40 °C (red) to 71.5 °C (pale yellow). (C) 2D heat map for Tus-GFP:Ter-lock complexes. (D) Pearson's r correlation between averaged Tm values (n = 3) obtained by visual analysis and by automatic Tm peak analysis of DSF-GTP curves (n = 184; data from free Tus-GFP and in complex with Ter, Ter-lock, oriC, oriC-lock). Red error bars: 95% confidence interval. | ||
Due to the prominent GFP peak starting at 72 °C,17 the melting curves (−dRFU/dT) were analyzed until 71.5 °C to detect the Tm peaks of Tus-GFP complexes – i.e. when the GFP signal is minimal in order to reduce the false Tm peak recognition rate. An arbitrary Tm value of 71.5 °C was automatically assigned to peaks at or above this temperature. This was only the case for Ter-induced stability of Tus-GFP at the lowest [KCl] (high affinity conditions) with the exception of TerF (Tm < 71.5 °C). The Tm values for the remaining Ter sites could be visually determined and were generally within 0.5 °C of the arbitrary value. Only for TerG and TerI, peaks at 73.6 °C and 73.1 °C, respectively, were missed by this method but could be obtained by visual examination. Overall, the data from the automatic peak recognition program correlated well with those obtained by visual determination of Tm for each curve (Fig. 2D). Out of 552 Tm peaks analyzed, only 24 were misidentified by the program, corresponding to an error rate of 4.3%. Out of these errors, ∼50% were due to the GFP peak being higher at 71.5 °C than the Tus-GFP peak, and the remaining errors were from unresolved peaks in the original curves. This error rate could probably be decreased by increasing the protein concentration and accordingly the signal.17
The 2D heat map of the 96-well plate provides a colour-coded representation of the experimental screen by transforming Tm values across the plate in a 2-colour gradient code, with the lowest Tm shown in dark red and the highest Tm shown in pale yellow (Fig. 2B and C). The profiles obtained for TerB and Ter-lockB were in agreement with previous data obtained under the same conditions using a visual analysis method,16 demonstrating the accuracy of the automatic Tm detection method and its utility for the analysis of high volumes of data.
3.2. Salt resistance of Tus–Ter and Tus–Ter-lock using a fast 2D DSF-GTP screen
The salt dependence of Tus-GFP in complex with ten Ter sites, their Ter-lock analogues and a non-specific DNA (oriC), was examined in the presence of [KCl] ranging from 8.5–350 mM as previously described for TerB and Ter-lockB17 using our improved HT DSF-GTP (Fig. 3). The Ter-lock oligonucleotides used in this study are partially single-stranded at their non-permissive side to allow the C(6) to bind into the cytosine-binding pocket of Tus, thus both creating new sequence specific interactions and reducing the overall number of non-specific electrostatic interactions (Fig. 3A, B and 4). When the salt dependence of complex stability of Tus-GFP:TerB was previously compared to Tus-GFP:Ter-lockB the stability curves crossed, suggesting that the difference in their slopes was the result of TT-lock formation.17 Here we compared the salt dependence of the remaining Ter with their Ter-lock species in complex with Tus-GFP to determine if this convenient and fast approach using HT DSF-GTP was able to detect the subtle differences in binding behavior of these species.![Effect of ionic strength on Tus-GFP in complex with Ter sites or their Ter-lock analogues. Representative sequences and structures of TerA (A) and Ter-lockA (B). Sequences in blue were added to all DNA sequences to increase their melting temperature. Stability curves of Tmvs. [KCl] (8.5–350 mM) for Tus-GFP:Ter (C) and Tus-GFP:Ter-lock (D). The curves obtained for TerA–E and TerG (C) and their respective Ter-locks (D) are almost identical and are represented as a single green square symbol in both panels. oriC and oriC-lock:Tus-GFP in complex with non-specific DNA. (E) Stability curves were organized in pairs of Tus-GFP:Ter (blue) vs. Tus-GFP:Ter-lock (red; tt) to highlight their differences (n = 3).](/image/article/2013/MB/c3mb70426b/c3mb70426b-f3.gif) | ||
| Fig. 3 Effect of ionic strength on Tus-GFP in complex with Ter sites or their Ter-lock analogues. Representative sequences and structures of TerA (A) and Ter-lockA (B). Sequences in blue were added to all DNA sequences to increase their melting temperature. Stability curves of Tmvs. [KCl] (8.5–350 mM) for Tus-GFP:Ter (C) and Tus-GFP:Ter-lock (D). The curves obtained for TerA–E and TerG (C) and their respective Ter-locks (D) are almost identical and are represented as a single green square symbol in both panels. oriC and oriC-lock:Tus-GFP in complex with non-specific DNA. (E) Stability curves were organized in pairs of Tus-GFP:Ter (blue) vs. Tus-GFP:Ter-lock (red; tt) to highlight their differences (n = 3). | ||
 | ||
| Fig. 4 Protein–DNA interactions at the non-permissive face of Tus. (A) TerA sequence. (B) Ter-lockA sequence. (C) Details of Tus–TerA interactions at the non-permissive face of Tus. (D) Interactions at the non-permissive face of Tus–Ter-lockA. Diagrams were adapted from NUCPLOT29 maps obtained with structural coordinates of PDB2I05 (Tus–TerA) and PDB2I06 (Tus–Ter-lockA). Boxed residues represent known interactions that were omitted by NUCPLOT. The shaded area (D) highlights the structures and interactions that are absent in our Tus–Ter-lock complexes due to the design of Ter-lock oligonucleotides used in this study. The C(6) involved in lock formation is represented in orange. | ||
As previously observed, the Tm of free Tus-GFP increased moderately and gradually with increasing [KCl] (Fig. 3C–E),17 indicating that ions bind and stabilize the protein.5 The non-specific oriC and oriC-lock increased the stability of Tus-GFP only at low ionic strength (i.e. < 150 mM [KCl]) resulting in a ΔTm > +10 °C at 8.5 mM [KCl] (Fig. 3C and D). The data highlight that significant intermolecular electrostatic interactions can only occur <150 mM [KCl] between Tus and non-specific DNA. When bound to the strong TerA–E and G, the Tm of Tus-GFP shifted up to +28 °C at 8.5 mM [KCl]. For these Ter sites, the Tm of Tus-GFP decreased gradually with increasing [KCl] due to the gradual breaking of intermolecular electrostatic interactions in the Tus-GFP:Ter complexes (Fig. 3C). The salt dependence curves obtained for all strong binders (TerA–E and G)16 were similar in shape – i.e. similar slopes, amplitudes and maximal Tm (Fig. 3C and Table 1) – suggesting that essentially the same ionic bonds are broken in these complexes. TerG binding resulted in the highest Tm at 8.5 mM [KCl], indicating that additional or stronger electrostatic interactions may occur in the Tus–TerG complex (Fig. 3E). As expected, the strong TerA–E and G induced a larger thermal shift than the moderate binders TerH–J at almost all salt concentrations, reflecting the higher affinity of Tus for these strong binders (Fig. 3C). Within the weak binders, TerI was the only one to be as stabilizing as the strong binders, but only at 8.5 mM [KCl] (Fig. 3C).
| Ligand | A | B | C | D | E | Fa | G | H | I | J | oriCb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Slopes (± SD) were obtained from linear regression of Tm values in the linear portion of the curve. The slopes of all Ter and Ter-lock curves were taken between 150 and 250 mM [KCl] exept for TerF, oriC and their respective lock analogues.a TerF and Ter-lockF were analysed between 100 and 200 mM [KCl].b oriC and oriC-lock were analyzed between 8.5 and 100 mM [KCl]. The max and min Tm values are the Tm at the lowest and highest [KCl], respectively, and the amplitude is the difference between these two values. | |||||||||||
| Ter | −0.085 | −0.082 | −0.079 | −0.081 | −0.087 | −0.094 | −0.085 | −0.087 | −0.092 | −0.095 | −0.092 |
| Slope | (0.005) | (0.007) | (0.006) | (0.004) | (0.003) | (0.005) | (0.006) | (0.005) | (0.004) | (0.006) | (0.008) |
| Max Tm | 71.4 | 71.5 | 71.6 | 71.5 | 71.3 | 68.4 | 73.6 | 71.7 | 73.1 | 71.6 | 57.4 |
| Min Tm | 49.8 | 50.1 | 49 | 48.8 | 48.4 | 44.6 | 49 | 46.3 | 46.4 | 45.6 | 44.6 |
| Amplitude (°C) | 21.6 | 21.4 | 22.6 | 22.7 | 22.9 | 23.8 | 24.6 | 25.4 | 26.7 | 26.0 | 12.8 |
| Ter-lock | −0.057 | −0.058 | −0.058 | −0.061 | −0.057 | −0.091 | −0.063 | −0.067 | −0.075 | −0.066 | −0.10 |
| Slope | (0.004) | (0.004) | (0.007) | (0.003) | (0.004) | (0.004) | (0.003) | (0.003) | (0.005) | (0.005) | (0.007) |
| Max Tm | 68.0 | 68.0 | 68.5 | 67.9 | 67.7 | 61.6 | 69.4 | 65.5 | 65.6 | 65.2 | 57.0 |
| Min Tm | 52.5 | 52.9 | 52.0 | 51.7 | 51.2 | 44.8 | 51.5 | 46.7 | 46.6 | 47.3 | 44.6 |
| Amplitude (°C) | 15.5 | 15.1 | 16.5 | 16.2 | 16.5 | 16.8 | 17.9 | 18.8 | 19.0 | 17.9 | 12.4 |
Ter species were systematically more stabilizing than their Ter-lock analogues in low salt. This phenomenon has previously been proposed to be due to missing base-specific and electrostatic interactions between G(6)/A(5) and the R198 residue of Tus in the partially single stranded Ter-locks compared to the Ter species.16 The R198 residue forms polar and Van der Waals contacts with the deoxyribose moiety of A(5) and G(6) in Ter species and a water-mediated ionic interaction with the phosphate group of G(6).12 It also forms specific hydrogen bonds with these two bases (Fig. 4C) and thus contributes significantly to the overall stability of the complex.20 The R198A Tus mutant has a 150-fold reduced binding affinity for TerB and a lower affinity for non-specific DNA due to its major impact on the association rate constant (ka), demonstrating the significant contribution of this residue to complex formation rather than complex stability.20 The higher stability induced by Ter compared to Ter-lock species in low salt is therefore mainly the result of a decreased association rate constant (ka) for the Tus–Ter-lock complexes. In high salt, ionic contacts are weakened, reducing the difference in ka between the two species and making the effect of the TT-lock apparent, at least for the strong TT-lock-forming sites.
A shouldering effect was observed in the low-salt part of the curves that was more prominent for the strong TerA–E and G than the remaining Ter sites (Fig. 3E). A steeper negative slope and larger amplitude were observed for Ter species than for their Ter-lock analogues (Table 1), resulting in the crossing of Ter and Ter-lock curves between 150–200 mM [KCl] for most TT-lock-forming Ter sites (Fig. 3E). This trend immediately suggests a larger contribution of electrostatic interactions in Tus–Ter complexes and the presence of additional specific interactions in Tus–Ter-lock complexes that increase their salt resistance. Ionic strength affects both specific and non-specific interactions in a protein–DNA complex. However, specific interactions are less severely affected by salt than non-specific interactions.2,23–26 In high salt, the major factor increasing the resistance of Tus–Ter-lock to thermal denaturation is therefore the formation of the TT-lock. Above 150 mM [KCl], when significantly less electrostatic interactions contribute to the stability of the complexes (i.e. no binding to non-specific oriC), the contribution of the TT-lock is sufficient to overcome and/or surpass the loss of both electrostatic and specific interactions with the nucleotides missing at the non-permissive face of the Ter-lockA–E and G species. This is reflected by the crossing of Ter and Ter-lock curves <200 mM [KCl] for strong TT-lock-forming species.
Ter-lockF was the least stabilizing species after TerF. In fact, TerF and Ter-lockF curves were essentially parallel and did not cross (steepest slopes of −0.94 ± 0.005 and −0.091 ± 0.004 respectively), confirming that TerF is the weakest binder and does not form a TT-lock (Table 1 and Fig. 3E).16 No gain in thermal stability could be observed for Tus-GFP in complex with TerF and Ter-lockF at [KCl] > 250 mM and 300 mM respectively. The lower stabilization of Tus-GFP with Ter-lockF is probably due to the absence of the interaction between R198 and the G(6) phosphate group (Fig. 4). The low affinity of TerF for Tus (previously determined by SPR) raised questions about the biological significance of TerF.16 The comparison of the TerF curve with the non-specific oriC curve demonstrates that TerF has maintained some specificity for Tus (Fig. 3C and D). Indeed, at physiological concentrations (∼150 mM [KCl]), oriC did not significantly stabilize Tus-GFP, whereas TerF induced a thermal shift of +12.5 °C. These data show that TerF could still act as a weak pausing site despite its low affinity and its inability to form a TT-lock.
The Tus-GFP stability curves obtained for TerH–J and their Ter-locks were quite irregular, revealing differences in their electrostatic and specific contributions to Tus-GFP binding. The slopes obtained for TerI and J were very steep (Table 1) compared to TerH. This is probably due to the loss of a specific interaction with T(9), which is mutated to an adenine in TerI and J (cf.Fig. 1B and 4). Within this group, TerI binding to Tus-GFP resulted in the highest Tm at 8.5 mM [KCl] suggesting that additional small cooperative electrostatic interactions may occur in the Tus–TerI complex (Fig. 3C). Although TerJ was most susceptible to ionic strength within this group – suggesting that it forms less specific interactions – Ter-lockJ was able to significantly stabilize Tus-GFP and confer a stronger resistance to ionic strength than Ter-lockI and H (cf. Min Tm values in Table 1). Indeed, the stability curves of Ter-lockJ and TerJ cross at ∼200 mM [KCl] whereas the curves obtained for TerI and Ter-lockI, and for TerH and Ter-lockH, cross at ∼300 and ∼250 mM [KCl] respectively (Fig. 3E). Ter-lockI produced the steepest slope of all TT-lock-forming Ter-lock species. This was surprising given that TerH cannot form a significant TT-lock whereas TerI can form a moderate TT-lock.16 While not conclusive, it could be speculated that as TerI has a reduced number of specific interactions compared to TerH it is more strongly affected by ionic strength.
Overall, the curves obtained with our new approach provided useful comparative information about the relative distribution of specific and electrostatic interactions occurring in the different complexes. Indeed, the comparison of the parameters of the curves such as steepness of the slopes as well as their minimal (min) and maximal (max) Tm in the range of [KCl] tested highlights the differences in ionic and specific interactions occurring in these complexes. The max Tm represents the total contribution of both ionic and specific interactions in the complex at low salt. The ionic interactions are gradually broken when [KCl] increases, indicating that complexes with a comparatively higher min Tm at high [KCl] indicate that they form more specific interactions than species with lower min Tm. Here, the differences in slopes and crossing points of the curves obtained for a given Tus-GFP:Ter and its Tus-GFP:Ter-lock complex correlate well with an increase in specific interactions resulting from TT-lock formation (Fig. 3). This approach could be very valuable for mutational studies to rapidly identify the ionic and specific interactions occurring in protein–DNA complexes.
3.3. Dissecting the ionic and specific contributions in Tus–Ter and Tus–Ter-lock
The number of ionic contacts N and the sequence-specific affinity parameter Kspe were determined for Tus-GFP with a subset of representative Ter sites and their respective Ter-lock variants; i.e. the strong TerB, the weak TerF, and the moderate TerJ. For this, Kobs values were determined at various [KCl] as previously described (Table S1, ESI†)17 to determine the slopes SKA = dlog(1/Kobs)/dlog[KCl]. Within the range of [KCl] tested, the Kobs obtained with DSF-GTP reflected the KD previously obtained by SPR for these species.16 To calculate N, SKA values were divided by 0.88, a factor previously used for the Tus–TerB complex in the same buffer.20 The SPR study by Neylon et al. revealed that 13 cations were displaced upon binding of Tus to TerB. Here, using our DSF-GTP we obtained N = 11 (Fig. 5). This number correlates better with the fact that there are only eight direct ionic contacts12,20 between lysine or arginine residues and DNA phosphate groups observed in the crystal structure, and that the maximum number of direct and indirect ionic contacts that could potentially form in Tus–TerB complex is 14.20 The N-values that we obtained for the 6 species ranged between 10 and 13 (Fig. 5B). As expected, the SKA values obtained for Tus-GFP:TerB and Tus-GFP:TerF were systematically steeper than for their respective Tus-GFP:Ter-lock variant suggesting that only one ionic contact was missing in these complexes. This is most likely due to the missing electrostatic interaction involving R198 in the partially single stranded Ter-lock species (Fig. 4). Interestingly, Tus-GFP:TerJ and Tus-GFP:Ter-lockJ have the same N value of 13 suggesting that more ionic contacts are formed in these complexes than in Tus-GFP:TerF (N = 12) and Tus-GFP:TerB (N = 11). It also suggests that the missing electrostatic interaction involving R198 is not occurring in Tus–TerJ and that additional ionic interactions can occur in these complexes. It can therefore be argued that the polar base-specific contacts that are broken upon binding of Tus to the moderate and weak Ter binders could rearrange to form new direct or water-mediated electrostatic interactions.![Ionic and specific contributions in Tus-GFP:Ter and Tus-GFP:Ter-lock. (A) Log–log plot of Tus-GFP:Ter and Tus-GFP:Ter-lock DNA binding constants (KA = 1/Kobs) as a function of [KCl]. (B) Determination of N and Kspe values for Tus-GFP in complex with TerB, F and J and their Ter-lock variants. SD: standard deviations (n = 2).](/image/article/2013/MB/c3mb70426b/c3mb70426b-f5.gif) | ||
| Fig. 5 Ionic and specific contributions in Tus-GFP:Ter and Tus-GFP:Ter-lock. (A) Log–log plot of Tus-GFP:Ter and Tus-GFP:Ter-lock DNA binding constants (KA = 1/Kobs) as a function of [KCl]. (B) Determination of N and Kspe values for Tus-GFP in complex with TerB, F and J and their Ter-lock variants. SD: standard deviations (n = 2). | ||
The relative values of KA (i.e. 1/Kobs) at any given [KCl] indicate the contribution of the specific component Kspe and the ionic component to the free energy of binding. As a result, when [KCl] = 1 M, the extrapolated KA = Kspe. The Kspe values obtained for Tus-GFP in complex with the three tested Ter and Ter-lock pairs are in the following order: Kspe for Ter-lockB ≫ TerB ≫Ter-lockJ > Ter-lockF, TerJ, TerF (Fig. 5) in reasonable agreement with previous data.16 The Kspe obtained with Ter-lockF, TerJ and TerF were very low, highlighting that when in complex with Tus these species form interactions that are mostly ionic. Although, caution must be taken when interpreting these data as the standard deviations are very high in these experiments. Most significant was the 10-fold higher Kspe for Tus-GFP:Ter-lockB than for Tus-GFP:TerB highlighting the specific contribution of TT-lock formation to overall complex stability.18 As expected, no significant difference in Kspe was observed between Tus-GFP:Ter-lockF and Tus-GFP:TerF, confirming that Ter-lockF cannot induce formation of a TT-lock.16 Finally, a 3-fold higher Kspe was observed for Tus-GFP:Ter-lockJ than for Tus-GFP:TerJ, indicating that the moderate Ter sites are not able to form a strong TT-lock.
4. Conclusion
This is the first comprehensive study examining the effect of ionic strength on the primary Tus–Ter and Tus–Ter-lock complexes using our HT DSF-GTP assay. The data revealed that the major factor increasing the resistance of Tus–Ter-lock complexes to thermal denaturation in high-salt is the formation of the TT-lock.18 This was supported by a 10-fold higher Kspe for Tus-GFP:Ter-lockB than for Tus-GFP:TerB. The data were readily analyzed using a new automatic Tm peak recognition protocol that we adapted to the DSF-GTP assay. The system is very accurate (96% of Tm peaks recognized) with most of the imprecision occurring >71.5 °C. Using this 2D screen format, i.e. salt vs. ligand, we were able to measure and analyze the effect of 8 [KCl] on 11 different Tus-GFP:DNA complexes and free Tus-GFP simultaneously in a 96 well format and in less than 2 hours. It is important to note that these data could not have been obtained by SPR due to instrumental and surface limitations. Taken together, these results illustrate that electrostatic interactions play an important role in the Tus–Ter complex formation and its stability. The system was able to detect subtle differences in overall Tus–Ter stabilization that could be attributed to the differences in specific and non-specific interactions occurring between Tus and the various Ter sites and their respective TT-lock forming capacity. The weakest TerF is the most susceptible to ionic strength followed by TerJ, TerI and TerH. TerA–C are the most specific sites based on their stabilizing effects at all salt concentrations, and Ter-lockB is the most salt-resistant site. Finally, we are confident that our fast 2D DSF-GTP screen approach will provide an invaluable comparative tool to study and decipher the mode of binding of other DNA-binding proteins and to examine the effect of mutations on complex stability at both DNA and protein levels.Acknowledgements
P.M.S. acknowledges support from the Smart Futures Fund of the Queensland Government, NIRAP. M.J.J.M. was supported by a JCU postgraduate research scholarship.References
- M. T. Record Jr., C. F. Anderson and T. M. Lohman, Q. Rev. Biophys., 1978, 11, 103–178 CrossRef CAS.
- M. T. Record Jr., J. H. Ha and M. A. Fisher, Methods Enzymol., 1991, 208, 291–343 CAS.
- G. S. Manning, Q. Rev. Biophys., 1978, 11, 179–246 CrossRef CAS.
- M. T. Record Jr., M. L. Lohman and P. De Haseth, J. Mol. Biol., 1976, 107, 145–158 CrossRef CAS.
- T. T. Waldron, G. L. Schrift and K. P. Murphy, J. Mol. Biol., 2005, 346, 895–905 CrossRef CAS PubMed.
- P. L. Privalov, A. I. Dragan and C. Crane-Robinson, Nucleic Acids Res., 2011, 39, 2483–2491 CrossRef CAS PubMed.
- D. Bastia and S. K. Singh, Bioarchitect, 2011, 1, 24–28 CrossRef PubMed.
- D. Bastia, S. Zzaman, G. Krings, M. Saxena, X. Peng and M. M. Greenberg, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 12831–12836 CrossRef CAS PubMed.
- F. F. Coskun-Ari and T. M. Hill, J. Biol. Chem., 1997, 272, 26448–26456 CrossRef CAS PubMed.
- D. B. Dahdah, I. Morin, M. J. Moreau, N. E. Dixon and P. M. Schaeffer, Chem. Commun., 2009, 3050–3052 RSC.
- I. G. Duggin and S. D. Bell, J. Mol. Biol., 2009, 387, 532–539 CrossRef CAS PubMed.
- K. Kamada, T. Horiuchi, K. Ohsumi, N. Shimamoto and K. Morikawa, Nature, 1996, 383, 598–603 CrossRef CAS PubMed.
- D. L. Kaplan, Curr. Biol., 2006, 16, R684–R686 CrossRef CAS PubMed.
- M. J. Moreau, I. Morin and P. M. Schaeffer, Mol. BioSyst., 2010, 6, 1285–1292 RSC.
- M. J. Moreau and P. M. Schaeffer, Analyst, 2012, 137, 4111–4113 RSC.
- M. J. Moreau and P. M. Schaeffer, Mol. BioSyst., 2012, 8, 2783–2791 RSC.
- M. J. J. Moreau, I. Morin, S. P. Askin, A. Cooper, N. J. Moreland, S. G. Vasudevan and P. M. Schaeffer, RSC Adv., 2012, 2, 11892–11900 RSC.
- M. D. Mulcair, P. M. Schaeffer, A. J. Oakley, H. F. Cross, C. Neylon, T. M. Hill and N. E. Dixon, Cell, 2006, 125, 1309–1319 CrossRef CAS PubMed.
- S. Mulugu, A. Potnis, Shamsuzzaman, J. Taylor, K. Alexander and D. Bastia, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 9569–9574 CrossRef CAS PubMed.
- C. Neylon, S. E. Brown, A. V. Kralicek, C. S. Miles, C. A. Love and N. E. Dixon, Biochemistry, 2000, 39, 11989–11999 CrossRef CAS PubMed.
- C. Neylon, A. V. Kralicek, T. M. Hill and N. E. Dixon, Microbiol. Mol. Biol. Rev., 2005, 69, 501–526 CrossRef CAS PubMed.
- P. M. Schaeffer, M. J. Headlam and N. E. Dixon, IUBMB Life, 2005, 57, 5–12 CrossRef CAS PubMed.
- L. E. Engler, K. K. Welch and L. Jen-Jacobson, J. Mol. Biol., 1997, 269, 82–101 CrossRef CAS PubMed.
- M. M. Garner and D. C. Rau, EMBO J., 1995, 14, 1257–1263 CAS.
- R. M. Saecker, in eLS, John Wiley & Sons, Ltd., 2001 Search PubMed.
- N. Y. Sidorova and D. C. Rau, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 12272–12277 CrossRef CAS.
- H. Seitz, C. Weigel and W. Messer, Mol. Microbiol., 2000, 37, 1270–1279 CrossRef CAS.
- M. Valens, S. Penaud, M. Rossignol, F. Cornet and F. Boccard, EMBO J., 2004, 23, 4330–4341 CrossRef CAS PubMed.
- N. M. Luscombe, R. A. Laskowski and J. M. Thornton, Nucleic Acids Res., 1997, 25, 4940–4945 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3mb70426b |
| ‡ School of Pharmacy and Molecular Sciences, James Cook University, DB21 James Cook Drive, Townsville, QLD 4811, Australia. E-mail: patrick.schaeffer@jcu.edu.au; Fax: +61 (0)7 4781 6078; Tel: +61 (0)7 4781 4448. |
| This journal is © The Royal Society of Chemistry 2013 |
