NMR-based metabolomics and LC-MS/MS quantification reveal metal-specific tolerance and redox homeostasis in Chlorella vulgaris†
Wenlin
Zhang
ab,
Nicole G. J.
Tan
ab and
Sam F. Y.
Li
*abc
aDepartment of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543. E-mail: chmlifys@nus.edu.sg; Fax: +65 6779 1691; Tel: +65 6516 2681
bNUS Environmental Research Institute, National University of Singapore, 5A Engineering Drive 1, Singapore 117411
cSchool of Environment and Energy, Peking University Shenzhen Graduate School, Shenzhen, People's Republic of China 518055
First published on 28th October 2013
Abstract
Live green algae are promising candidates for phytoremediation, but a suitable algal species which bio-accumulates high concentrations of heavy metals, and survives well in industrial water is yet to be identified. Potential metabolic engineering may be applied to improve algal phytoremediation performance, but the metal tolerance and bioaccumulation mechanisms in green algae have to be first fully understood. In this study, NMR-based metabolomics was used to study the effect of different metal species (copper, cadmium and lead) and metal concentrations in green microalgae, Chlorella vulgaris. High Cu concentrations influenced substantial decrease in organic osmolytes (betaine and glycerophosphocholine), which indicated Cu-induced redox imbalance. Accompanying redox imbalance, growth inhibition and photosynthesis impairments in Cu-spiked C. vulgaris revealed a clear relationship between Cu toxicity and redox homeostasis. As these metabolic changes were less prominent in Cd and Pb-spiked cultures, we inferred metal-specific toxicity in C. vulgaris, where redox active Cu2+ is more potent than non-redox active Cd2+ and Pb2+ in causing redox imbalance. Subsequently, ICP-MS and LC-MS/MS quantification shed light on the metal-specific bioaccumulation and detoxification mechanisms. The metal bioconcentration factor (BCF) correlated well with the phytochelatin (PC) content in Cu and Cd-spiked C. vulgaris biomass. High BCF and PC levels with increasing Cu and Cd exposure concentrations indicated that PCs played a significant role in Cu and Cd bioaccumulation and detoxification. In contrast, the undetectable PC levels in Pb-spiked cultures despite high Pb BCF suggest an alternative detoxification mechanism for Pb: either by passive absorption to the algal cell wall or interaction with glutathione (GSH).
Introduction
Heavy metals in the environment may be essential or detrimental to living organisms. While most heavy metals are biologically non-essential for plants, and usually toxic at low concentrations, some trace metals such as copper, iron and zinc are essential for organism growth and development. However, these trace elements are potentially toxic when present in excess amounts. Over the years of industrialization, the emission of toxic heavy metals in urban areas is mainly attributed to anthropogenic activities. One of the major sources of heavy metal contamination in soils is the landfill of ash residues from coal combustion. Treated sewage discharges and runoffs from metal-refining industrial facilities are primary sources of heavy metal emission in water.1,2 Leaching of non-biodegradable toxic metals into water and soils not only has detrimental effects on the ecosystem, but also poses potential health risks to animals and humans. The inconvenient truth of metal pollution has sparked authorities to impose regulations on metal waste disposal. Given the growing importance of corporate social responsibility in today's modern economy, companies are involved in remediation of contaminated sites. As cleaning up heavily contaminated industrial areas is a costly endeavor,3 companies are in search of cheaper and more efficient remediation methods.In recent years, phytoremediation, the use of plants to remove pollutants from the environment, has gained recognition as an alternative green and cost-efficient method. Vascular plant species that not only exhibit tolerance to high metal concentrations, but also hyperaccumulate specific metals in their roots are utilized to clean up metal contaminated soil.4,5 An attractive aspect of phytoremediation, apart from its low maintenance cost, is that it is a carbon dioxide neutral technology. However, the slow growth of higher plant species and low metal uptake efficiency are some disadvantages of phytoremediation.3 Furthermore, the technology is widely applied for remediating metal contaminated soil rather than wastewater.
Green algae are promising candidates for phytoremediation as they are widely available in temperate and tropical regions, and different species can be found in both freshwater and marine water. At present, dried algal biomass has been utilized as heavy metal biosorbent in wastewater, where heavy metals are bound to dead algal cell walls.6,7 Dried biomass has greater binding capacity than live algal cultures,8,9 but unlike live algae, the binding capacity of dried biomass cannot be further improved through genetic or metabolic engineering approaches. Although the use of live algae has clear advantages over dried algal biomass, the key challenge of using live algae for phytoremediation is the search for a suitable algal species. An ideal algal species has to bioaccumulate high concentrations of heavy metals, and survive well in metal contaminated industrial water. The metal tolerance and bioaccumulation mechanisms in green algae have to be fully understood, prior to the development of potential engineering approaches to improve performance of algae in bioconcentration of heavy metals from industrial wastewater.
At present, many studies have reported heavy metal-induced toxicity in different algal species, with most demonstrating significant biochemical responses such as changes in free protein, carbohydrate and amino acid contents in algal cultures spiked with metals.10,11 However, these studies usually use different techniques to quantify respective variables, thus more samples and time are required in the analysis. In this study, we attempt to shed light on the heavy metal toxicity and tolerance in green microalgae (Chlorella vulgaris) using plant metabolomics, which enables simultaneous detection and quantification of metabolites. Nuclear magnetic resonance spectroscopy (NMR) and mass spectroscopy (MS) are generally the preferred analytical methods for plant metabolomics, as the data obtained from these analyses are more reproducible.12,13 Although MS-based detection has higher sensitivity than NMR, we believe that these methods are complementary to each other. Since NMR facilitates high throughput detection of primary and secondary metabolites, and has a simple sample preparation procedure, we have selected NMR as the analytical tool for primary macro-level analysis of the C. vulgaris metabolome.
Several genomic and proteomic studies revealed that efficient metal uptake and transportation across to the plant cytoplasm, followed by active antioxidant defense systems are crucial mechanisms to mediate metal toxicity.14 Metal toxicity and tolerance is also highly dependent on plants' antioxidant capacity and redox homeostasis.14 To have a holistic overview of the effect of heavy metals on green microalgae, we will take on a metabolomics approach to gain insight into the metabolic responses of C. vulgaris as a consequence of metal stress. Metabolites that are responsible for regulating phytotoxicity will also be simultaneously determined. Although investigation into the consequences of metal exposure in plants using NMR is ongoing for over a decade, most studies focus on either profiling of the metal-spiked plant metabolome, or identification of metal stress biomarkers, but rarely report on the metal tolerance mechanisms. Hence we aimed to gain a more comprehensive understanding of C. vulgaris toxicity and tolerance towards different concentrations of common metal pollutants using NMR-based metabolomics.
Three divalent metal ions, copper (Cu2+), cadmium (Cd2+) and lead (Pb2+), were selected for the study due to their persistent and biomagnification nature in food chains. Cd and Pb are among the most hazardous heavy metals in the environment. Being non-essential metals for plant metabolism, plants tend to exhibit a varied degree of phytotoxicity such as adverse effects to photosynthesis, when exposed to even low concentrations of these heavy metals.15,16 Although Cu is essential for plant growth activities such as CO2 assimilation, excess amount of Cu is usually toxic to plants.17 Redox active Cu, and non-redox active Cd and Pb are known to either directly or indirectly induce cellular damage by initiating the formation of reactive oxygen species (ROS) such as hydroxyl radicals (OH˙) and hydrogen peroxide (H2O2). Thus, understanding the metal-influenced redox homeostasis is a key step toward elucidating the metal tolerance mechanisms in green algae.18,191H NMR metabolite profiling complemented with multivariate data analysis will be used to reveal the substantial variability in C. vulgaris metabolome upon exposure to different metal treatments. The metabolites (biomarkers) which influenced discrimination of the sample classes will then be identified, subjected to statistical tests, and used in the elucidation of the metal-induced responses in C. vulgaris. The NMR-based metabolomic analysis will be correlated with metal uptake capacity determined using inductively coupled plasma-mass spectrometry (ICP-MS) and changes in glutathione and phytochelatin levels using liquid chromatography-tandem mass spectrometry (LC-MS/MS) to gain insights into the metal bioaccumulation and detoxification mechanisms in C. vulgaris.
Materials and methods
Chemicals
All growth medium and nutrient solutions were prepared using deionised water. All eluents, extraction buffers, and standard solutions were prepared with ultrapure water (Smart2Pure, Thermo Scientific TKA). Nitric acid (65% HNO3, trace analysis grade) was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Cadmium (Cd) and lead (Pb) ICP standard was purchased from Inorganic Ventures (Christiansburg, VA, USA). Copper (Cu), germanium (Ge), iridium (Ir) and rhodium (Rh) ICP standards were purchased from High-Purity Standards (Charleston, SC, USA). Copper chloride dihydrate (CuCl2·2H2O), cadmium chloride hemi(pentahydrate) (CdCl2·2½H2O), lead nitrate (Pb(NO3)2), 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS), diethylene triamine pentaacetic acid (DTPA), formic acid, reduced (GSH) and oxidized (GSSG) glutathione, N-acetyl-L-cysteine (NAC) and tris(2-carboxyethyl)phosphine (TCEP) were purchased from Sigma-Aldrich (St Louis, MO, USA). Phytochelatins (γ-Glu-Cys)2-Gly and (γ-Glu-Cys)3-Gly (PC2, PC3) were purchased from AnaSpec, Inc. (Fremont, CA, USA). Deuterium oxide (D2O) was purchased from Cambridge Isotope Laboratories (Andover, MA, USA).Cell cultivation
A total of 21 biological replicates of freshwater green microalgae, C. vulgaris (Carolina Biological, NC, USA) were cultivated under sterile controlled conditions (photoperiod of 18 h day/6 h night, 24 °C) in Bold's basal medium (2 L per culture).20,21 The C. vulgaris cell density was determined using both haemocytometer and UV spectrometer (OD442.5). Cultures grown to the early stationary phase (∼3 × 107 cells per mL) were spiked with different concentrations (0, 10, 50, 100, 200 & 500 μM) of CuCl2, CdCl2 and Pb(NO3)2 solutions. The cultures were subsequently incubated using different metal treatments for 72 hours before the cells were rinsed in ultrapure water, collected by centrifugation and lyophilized overnight.NMR-based metabolomic studies
Dried algal biomass (40.00 ± 0.05 mg) was resuspended in cold aqueous methanol (20% methanol). The suspension was subjected to ultrasonication (30 min, 4 °C), and the cell debris was removed by centrifugation (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 15 min, 4 °C). The extraction procedure was repeated twice. The supernatant was pooled together and lyophilized overnight. Lyophilized samples were reconstituted in phosphate buffer (0.1 M, pH 7.4 with 10% D2O) with a chemical shift indicator (0.5 mM DSS). All spectra were acquired under automation on a 600 MHz NMR spectrometer (Premium Shielded Narrow Bore, Agilent Technologies, CA, USA). Each spectrum consisted of 128 scans of 16
000 rpm, 15 min, 4 °C). The extraction procedure was repeated twice. The supernatant was pooled together and lyophilized overnight. Lyophilized samples were reconstituted in phosphate buffer (0.1 M, pH 7.4 with 10% D2O) with a chemical shift indicator (0.5 mM DSS). All spectra were acquired under automation on a 600 MHz NMR spectrometer (Premium Shielded Narrow Bore, Agilent Technologies, CA, USA). Each spectrum consisted of 128 scans of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 384 data points in the frequency domain, and were collected using water suppression (relaxation delay of 2.00 s) followed by a Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence. The spectra were automatically Fourier transformed using an exponential window with a line broadening value of 0.5 Hz, phased and baseline corrected using Chenomx NMR suite 7.6 (Chenomx Inc., AB, Canada). 1H NMR chemical shifts in the spectra were referenced to the DSS methyl peak at δ 0.00.
384 data points in the frequency domain, and were collected using water suppression (relaxation delay of 2.00 s) followed by a Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence. The spectra were automatically Fourier transformed using an exponential window with a line broadening value of 0.5 Hz, phased and baseline corrected using Chenomx NMR suite 7.6 (Chenomx Inc., AB, Canada). 1H NMR chemical shifts in the spectra were referenced to the DSS methyl peak at δ 0.00.
The regions of δ 4.98–4.62 and δ 3.48–3.34 were excluded to eliminate the residual water and methanol signals, respectively. The integrated data were normalized to units of standardized area (sa), where 1 sa is the area under a theoretical DSS methyl peak at 0.5 mM, before the spectra were reduced to integrated regions or “bins” of equal width (0.04 ppm) between a chemical shift of −0.5 ppm and 9.50 ppm using Chenomx NMR suite 7.6. The processed data was imported into SIMCA-P 12.0 (Umetrics, Sweden) for multivariate data analysis. Pareto scaling was performed before principal components analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were modeled. Metabolites were assigned using the Chenomx Profiler 600 MHz database. Statistical significance analysis, one-way ANOVA followed by post hoc Tukey's Honest Significant Difference (HSD) test, performed using MetaboAnalyst platform22,23 (http://www.metaboanalyst.ca) was used to identify the regions that showed significant changes between various metal treatment groups compared to the control, or changes between metal concentration groups. 1H NMR correlation heatmaps for the different metal species were also generated using MetaboAnalyst. Lastly, a list of tentatively assigned metabolites was generated. The chemical shifts for each tentatively assigned metabolite were compared against the Chenomx 600 MHz standard library, the chemical shifts of metabolites in Madison Metabolomics Consortium Database24 (http://mmcd.nmrfam.wisc.edu/) and some published data.31–35
Elemental analysis
Dried algal biomass (10.00 ± 0.03 mg) was acid digested using microwave heating (15 min, 180 °C, ETHOS One, Milestone). After cooling, the samples were made up to 10 mL with ultrapure water. 2% (v/v) suprapure nitric acid was added to filtered growth medium (0.2 μm) and acid digested biomass, and diluted for analysis using ICP-MS (7700X, Agilent Technologies, CA, USA). The concentration of Cu, Cd and Pb present in medium and biomass were quantified using an external calibration curve plotted from seven multi-elemental solutions (0, 10, 20, 50, 100, 250, 500 μg L−1) prepared from single-element ICP-MS standards. A mixture of Rh, Ge, Ir (100 μg L−1) was used as the internal standard. The elements with the highest isotopic abundance and free from isobaric and polyatomic interference were selected as the analytical mass. The metal uptake capacity and bioconcentration factor25 were computed using eqn (1) and (2), where [M]M,I and [M]M,F represent the initial and final metal concentrations in the medium respectively and [M]B,F represents the metal content in algal biomass after different metal treatments.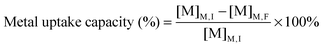 | (1) |
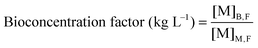 | (2) |
Quantification of biomarkers
The extraction procedure was modified from published protocol.26,27 The extracted compounds were separated using a C18 HPLC column (100 mm × 4.5 mm, 3.5 μm, 40 °C, Zorbax Plus) on a HPLC (Ultimate® 3000, Dionex, CA, USA) coupled with quadrupole-ion trap mass spectrometer (QTRAP® 5500, AB Sciex, DC, USA). Chromatography was performed on a 2–50% acetonitrile linear gradient at a flow rate of 0.5 mL min−1 for 10 min, followed by 50–2% acetonitrile linear gradient for 2 min. The final step gradient re-equilibrated the column to the initial run condition (2% acetonitrile) for 3 min. Both the organic and aqueous eluents contained 0.1% formic acid. All compounds were ionized in positive mode using electrospray ionization and detected using the following mass transition pairs: GSH (308 → 179, 308 → 162); GSSG (613 → 355, 613 → 231); PC2 (540 → 336, 540 → 233); PC3 (772 → 233, 772 → 179); NAC (164 → 122, 164 → 43). The concentrations of unknown compounds were quantified using an external calibration curve plotted from six standard mixture solutions (GSH, GSSG: 0, 100, 200, 500, 750, 1000 μg L−1; NAC, PC2, PC3: 0, 10, 20, 50, 75, 100 μg L−1). NAC (50 μg mL−1) was used as the internal standard to correct matrix effects and monitor possible abnormalities during the run. To prevent oxidation of the analytes, the reducing agent (TCEP) was added to the standard mixtures. The chromatograms were integrated using Analyst 1.5.1 (AB Sciex, DC, USA).Results and discussion
C. vulgaris cell growth under different metal treatments
The cell density of C. vulgaris exposed to various concentrations of essential (Cu) and non-essential (Cd & Pb) metals was monitored daily over a period of 72 h to determine the effect of heavy metals on C. vulgaris growth. Cultures spiked with 100 μM or lower CuCl2 concentrations showed significant growth over a period of 72 h, but growth inhibition was observed after C. vulgaris was exposed to 200 μM and 500 μM of CuCl2 for 72 h (Fig. S1A, ESI†). A similar growth pattern was observed for C. vulgaris treated with CdCl2. The growth rate of C. vulgaris was unaffected when the medium was spiked with 200 μM or lower CdCl2 concentrations, while growth inhibition was observed for culture spiked with 500 μM CdCl2 after being exposed for 48 h as evidenced by insignificant changes to the C. vulgaris cell density between 48 h and 72 h (Fig. S1B, ESI†). Interestingly, Pb(NO3)2 did not seem to influence C. vulgaris growth, as shown by the significant growth of cultures, even after exposure to high Pb(NO3)2 concentrations for 72 h (Fig. S1C, ESI†).The C. vulgaris growth trend indicated that green microalgae are more tolerant towards higher Cd and Pb concentrations than Cu over a period of 72 h. To explain why Cu exhibited greater influence on C. vulgaris growth as compared to Cd and Pb, we looked into how these heavy metals induce stress on the algal cells. Heavy metals are generally categorized into redox active and non-redox active metal groups. The redox active metals are directly involved in the production of reactive oxygen species (ROS) via Haber–Weiss and Fenton reactions.28 These ROS are responsible for lipid peroxidation, leading to cell damage and death. Such adverse effects on the cells are known as metal-induced oxidative stress. On the other hand, non-redox active heavy metals do not directly induce the formation of ROS, but rather chelate to the thiol-groups on active sites of antioxidant enzymes and glutathione. Binding of the non-redox active metals to the antioxidant enzymes lead to inactivation of the organism's antioxidant defense system, hence resulting in a redox imbalance and accumulation of ROS.14 As Cu2+, a redox active heavy metal, has a more direct effect on redox imbalance, we would expect Cu-spiked C. vulgaris to display greater metal-induced oxidative stress as compared to non-redox active heavy metals (Cd and Pb).
While we observed normal growth of C. vulgaris when exposed to high concentrations of Pb, other studies reported plant growth inhibition even at low metal concentrations.29,30 The discrepancy in toxic responses may be explained by the different activities of antioxidant enzymes, which could be stimulated, unaffected or suppressed in metal-stressed plants.14 The activity of antioxidant enzymes could be dependent on the plant species, metal ion, metal concentration and exposure duration. As these factors were among the variables of different studies, we would expect some differences in plant metal-induced toxic response. Furthermore, we believed that the high cell density used in this experiment contributed to the enhanced Pb tolerance. A large amount of dried algal biomass was required for metabolomic and elemental analysis. Instead of scaling up the culture volume, a rich nutrient medium (Bold's basal medium) was used, where an average cell density of 3 × 107 cells per mL was achieved in the early stationary phase. Hence, the cell density used for our metal toxicity and tolerance study is almost 100 times higher as compared to microalgae cultures used in other studies.29,30 As each plant cell plays a significant role in reducing the free metal ion availability through binding to the active sites on the algal cell wall and chelation with high affinity metal binding molecules, the higher cell density could mediate metal-induced toxicity more efficiently.
Metabolomic study of single metal tolerance in C. vulgaris
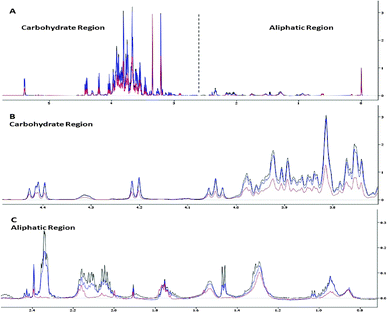
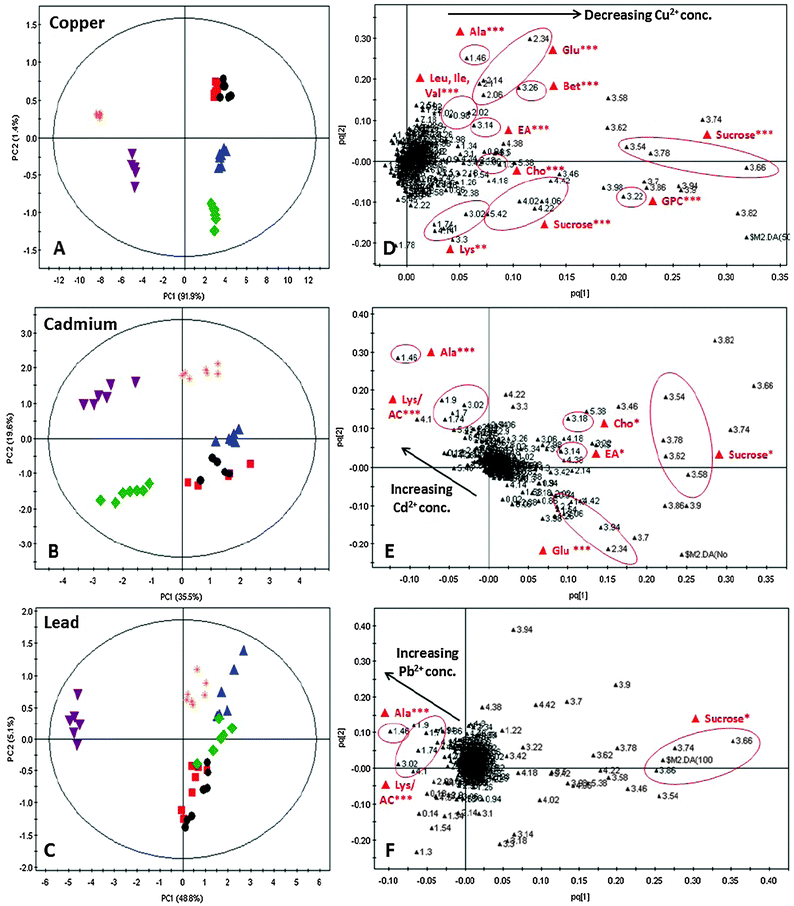
The C. vulgaris metabolome was examined to gain insights into the metal-specific tolerance response. The metabolomic studies were established using 1H NMR spectroscopy together with multivariate data analysis. Fig. 1 displays the 1H NMR spectra of C. vulgaris control polar extracts overlaid with cultures spiked with 50 μM and 500 μM CuCl2 respectively. The spectra show clear dominance and overlapping of signals in the carbohydrate regions which were mainly composed of sucrose, and well-defined signals in the aliphatic region which consisted of amino acids. Although some differences in the peak area at the carbohydrate and aliphatic regions were readily observed between control and metal-spiked samples, these visual inspections could not be used to accurately describe the metabolic changes between samples.A more reliable method, i.e. multivariate analysis, was thus used to assess the effect of different concentrations of Cu2+, Cd2+ and Pb2+ on the C. vulgaris metabolome. Principal components analysis (PCA) and orthogonal partial least square-discriminant analysis (OPLS-DA) are two commonly used multivariate analysis methods to reduce the dimensionality of the raw data into a few principal components which describe the maximum possible variation in the data. In the PCA scores plot, discrimination between different metal-treated sample classes was vaguely observed. Based on Hotelling's T2 with 95% confidence, several strong outliers were determined and eliminated from the PCA scores plot. Although better clustering was achieved in the OPLS-DA scores plot, the effect of individual metal concentrations could not be interpreted in the OPLS-DA scores plot as the results for different concentrations of each metal were severely overlapped (Fig. S2, ESI†). As such, OPLS-DA was carried out between Cu-spiked samples and untreated C. vulgaris in model A, Cd-spiked samples and untreated C. vulgaris in model B and Pb-spiked samples and untreated C vulgaris in model C (Fig. 2A–C). The cross-validated predictive ability Q2(cum) in models A, B and C were 73.9%, 64.6%, 44.2% respectively, and the total explained variance R2(cum) for models A, B and C were 97.6%, 83.1%, and 87.9% respectively.
The OPLS-DA scores plot showed that increasing the concentration of each metal affected the C. vulgaris metabolome. Tight clustering of low metal concentration classes together with the control sample class suggested that these concentrations were insufficient to induce a significant change in the metabolic response. In comparison, the high metal concentrations were separated from the control group in a concentration-dependent manner. Furthermore, it was observed that cultures exposed to Cu (Fig. 2A) were more differentiated than those of Cd and Pb (Fig. 2B and C) from the control group based on the first principal component (PC1). The OPLS-DA loadings plot for individual metal was projected to gain insights into the metabolites responsible for the discrimination pattern observed in Fig. 2A–C. Metabolites that showed significant differentiation (p < 0.001) in the OPLS-DA loadings plot (Fig. 2D–F) were identified and quantified against the NMR chemical shift indicator (DSS). Table 1 summarizes the list of tentative assignments of metabolites and their respective metabolic changes as a consequence of exposure to different metal species and metal concentrations. Changes in these metabolites upon exposure to different metal species and metal concentrations were analyzed and represented in terms of relative concentrations, in Fig. S3 (ESI†). Heatmap correlation of C. vulgaris polar extract 1H NMR spectra demonstrated that Cu-induced stress score correlated with carbohydrate and osmoprotectant as these metabolites were substantially depleted in cultures exposed to high Cu concentrations. On the other hand, Cd and Pb-induced stress scores were greatly influenced by acetate and alanine, as shown by the substantial increased acetate and alanine levels in cultures spiked with excess Cd and Pb (Fig. S4, ESI†).
| Metal treatments | Chemical shift (ppm) | Tentative assignment | Metabolic changes | Possible metabolic responses | Ref. |
|---|---|---|---|---|---|
| >200 μM CuCl2 spiked samples | 4.06(t), 4.22(d), 5.42(d) | Sucrose | ↓↓↓ | Photosynthesis impairment, affecting glycolysis and amino acid biosynthesis | 1, 2 |
| 1.48(d) | Alanine | ↓↓↓ | 1, 3 | ||
| 0.96(t) | Leucine | ↓↓↓ | 1, 3 | ||
| 0.94(t) | Isoleucine | ↓↓↓ | 1, 3 | ||
| 0.99(d), 1.04(d) | Valine | ↓↓↓ | 1, 3 | ||
| 2.13(m), 2.48(m) | Glutamate | ↓↓↓ | 3, 5 | ||
| 3.20(s) | Choline | ↓↓↓ | Cu-induced oxidative stress | 1, 3, 4 | |
| 3.37(d), 3.9(d) | Ethanolamine | ↓↓↓ | 5 | ||
| 3.21(s) | Glycerophosphocholine | ↓↓↓ | 4 | ||
| 3.26(s) | Betaine | ↓↓↓ | 1, 3, 4 | ||
| 100 μM CdCl2 spiked samples | 4.06(f), 4.22(d), 5.42(d) | Sucrose | ↓↓ | Cd-induced oxidative stress | 1, 2 |
| 3.21(s) | Glycerophosphocholine | ↓↓ | 4 | ||
| 3.26(s) | Betaine | ↓ | 1, 3, 4 | ||
| >200 μM CdCl2 & 500 μM Pb(NO3)2 spiked samples | 1.48(d) | Alanine | ↑↑↑ | Cd and Pb-induced hypoxic stress | 1, 3 |
| 1.92(s) | Acetate | ↑↑↑ | 5 | ||
| 2.13(m), 2.48(m) | Glutamate | ↓↓↓ | 3, 5 |
Metabolic responses of C. vulgaris as a consequence of redox-active Cu
Investigating the OPLS-DA loadings plots and heat map correlations revealed that C. vulgaris has different metabolic responses towards redox-active Cu and non-redox-active metals (Cd, Pb) (Fig. 2D–F). A significant decrease (p < 0.001) of sucrose and glycerophosphocholine in C. vulgaris exposed to greater than 200 μM CuCl2 suggested that excess amount of Cu2+ could result in photosynthesis impairment and also indirectly induced oxidative stress. Although Cu is essential for plant cellular metabolism, it is known to induce adverse effects on photosynthesis when present at higher concentrations.17 Photosynthesis not only produces carbohydrates, but also contributes significantly to amino acid biosynthesis. Intermediates of the carbohydrate metabolism can be utilized to synthesize various amino acids. For example, Ala, Val and Leu are biosynthesized from pyruvate, a key intermediate of aerobic glycolysis. The decreased carbohydrate content together with dominant amino acids such as alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile) and glutamate (Glu) when exposed to high CuCl2 concentrations in our metabolomic analysis confirmed that excess Cu2+ exposure led to photosynthesis impairment in C. vulgaris. Apart from pyruvate, glutamate (Glu) also plays a significant role in the biosynthesis of Ala, Val and Leu, which involved the transamination of the amino moiety from Glu to these amino acids. Lowered Glu content in cultures spiked with more than 200 μM CuCl2, possibly utilized for the biosynthesis of glutathione,36 could also attribute to the declined amino acid levels.Accompanying the decrease in glycerophosphocholine (GPC) content in C. vulgaris spiked with greater than 200 μM CuCl2 are ethanolamine, choline and betaine. Betaine and GPC are organic osmolytes responsible for osmoregulation and osmoprotection, while ethanolamine and choline are precursors for GPC and betaine biosynthesis. Generally, these organic osmolytes are accumulated in plants to combat abiotic stress such as salinity, extreme temperature and heavy metals.37,38 However, recent proteomic studies revealed inactivation of betaine aldehyde dehydrogenase (BADH) responsible for catalyzing oxidation of betaine aldehyde to glycine betaine,39 and neuropathy target esterase (NTE) responsible for GPC biosynthesis,40 by reactive oxygen species (ROS).41,42 These proteomic studies coincided with our metabolomic analysis, which suggested that the decreased betaine and GPC concentrations in C. vulgaris spiked with high Cu concentrations are biomarkers of Cu-induced oxidative stress. Although the ROS content in C. vulgaris biomass was not quantified in this experiment, Cu-induced activation of the algal antioxidant defense system as well as elevated lipid peroxidation were some of the common phenomena observed in other Cu-stressed studies.43–46 These studies justified our findings that ROS could have been accumulated in the presence of excess redox-active Cu2+, thus causing redox imbalance which led to oxidative stress. The substantial growth inhibition when the cultures were exposed to 200 and 500 μM CuCl2 further supported that C. vulgaris failed to achieve redox homeostasis in a high Cu environment. This led to metal-induced photosynthesis impairment and oxidative stress, where both conditions have adverse effects on cell growth and development. The Cu-induced metabolic changes and their respective effects on the metabolic pathway are summarized in Fig. S5A and B (ESI†).
Effects of non-redox active Cd and Pb on C. vulgaris metabolome
C. vulgaris exposed to Cd and Pb (non-redox active metals) did not undergo similar metabolic changes to Cu-spiked cultures (Fig. 2E and F). Significant decrease in the sucrose level was observed for C. vulgaris treated with 100 μM CdCl2 and 500 μM Pb(NO3)2 respectively. While the reduced carbohydrate content suggested that C. vulgaris was experiencing Cd-induced photosynthesis impairment when exposed to 100 μM CdCl2, the relative concentrations of sucrose in cultures exposed to more than 200 μM CdCl2 were comparable to control cultures (ESI,† Fig. S3B). This could indicate metal tolerance towards higher concentrations of Cd2+, which will be discussed in further detail in the following elemental analysis section. Apart from 100 μM CdCl2, none of the other non-redox metal concentrations were able to induce significant changes (p < 0.05) in GPC and betaine levels in C. vulgaris. Since we have discussed that GPC and betaine are biomarkers of indirect metal-induced oxidative stress, we could interpret these metabolic changes as Cd and Pb being less potent than Cu in inducing oxidative stress. The continuous C. vulgaris growth over a period of 72 h even at high Cd and Pb concentrations also indicated that C. vulgaris is more tolerant to Cd and Pb as compared to Cu.Another set of metabolic changes in C. vulgaris spiked with high concentrations of CdCl2 and Pb(NO3)2 include a substantial increment in Ala, acetate, and decrease in Glu (p < 0.001). A recent genomics study by Limami and co-workers47 demonstrated that the simultaneous accumulation of Ala and decreased Glu content in young Medicago truncatula seedlings was a consequence of oxygen deprivation during water-logging of the soil. This led us to a new research question of whether exposure to high Cd and Pb concentrations induced hypoxia in green microalgae. Most hypoxic responses in unicellular algae were reported based on Chlamydomonas reinhardtii, as it is considered a model organism for understanding the photosynthetic energy metabolism under anoxic conditions.48 Under oxygen deprived conditions, oxygen becomes limiting for oxidative phosphorylation, hence plant cells switch to alternative metabolic pathways to produce adenosine triphosphate (ATP). In order to maintain glycolysis under anoxic conditions, plants initiate ethanol or lactate fermentation from pyruvate to maintain NAD+ regeneration.49 However, the accumulation of lactate in plant cells could damage the cells by aggravating cytoplasm acidification, and acetaldehyde (intermediate of ethanol fermentation) could form chemically reactive acetaldehyde–protein adducts.47 To exclude the accumulation of lactate and acetaldehyde in the cells, excess pyruvate was converted to Ala and acetate. The elevated levels of Ala and acetate found in our metabolic study suggested that high CdCl2 and Pb(NO3)2 conditions could induce hypoxia. The decreased Glu level was likely utilized for alanine biosynthesis, where pyruvate reacts with Glu to form Ala and 2-oxoglutarate via alanine aminotransferase (AlaAT).
This hypothesis is supported by increased AlaAT activity observed in Lotus japonicus,50Arabidopsis thaliana,51 and Medicago truncatula47 under hypoxic stress. While studies have demonstrated the ability of different plant species to accumulate alanine under anoxic conditions,51,52 no prior studies have documented metal-induced hypoxic stress in plants. Hence, we could only hypothesize that high concentrations of non-redox active metals (Cd2+ and Pb2+) reduce oxygen availability. This was in agreement with the report of Wang and coworkers,53 where Chlorella pyrenoidosa experienced reduced net oxygen evolution rates and respiratory oxygen consumption rates in the presence of high Cd concentrations. Based on the metabolic changes observed from C. vulgaris exposed to high Cd and Pb, we gathered that these non-redox active metals were less capable of inducing oxidative stress. We also suggest for the first time from a metabolomic approach that high Cd and Pb could lead to hypoxic stress. The Cd and Pb-induced metabolic changes and respective effects on the metabolic pathway are summarized in Fig. S6 (ESI†).
Metal uptake and bioconcentration efficiency in C. vulgaris
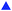
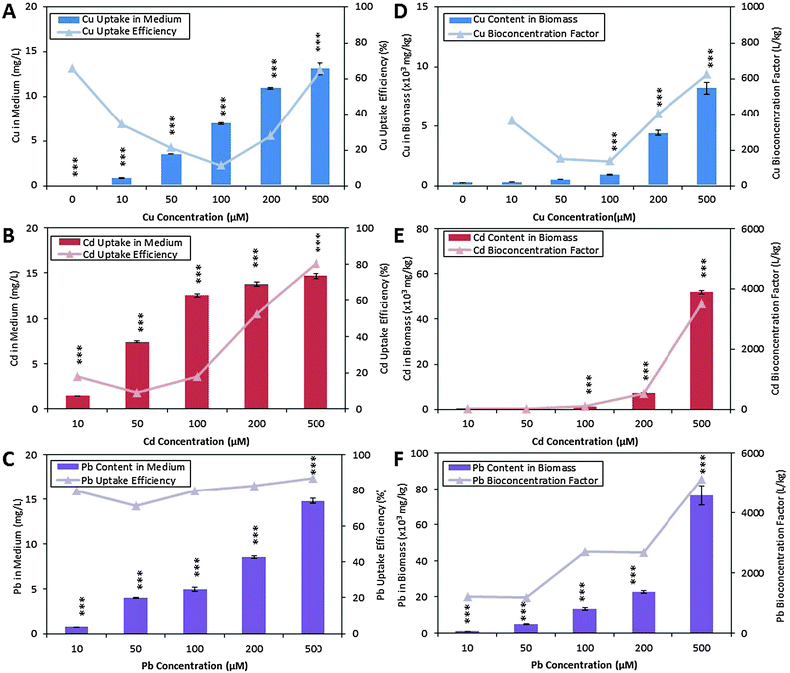
The metal contents in algal biomass were determined to confirm that the metabolic changes observed using 1H NMR spectroscopy were solely due to Cu, Cd and Pb toxicity and tolerance, and were not consequences of other abiotic stress factors like temperature and pH. The mean percentage recovery of Cu, Cd and Pb were 106.2%, 102.8% and 102.9%, respectively, and the limits of quantification of these metals were 1.67 μg L−1, 4.39 μg L−1 and 1.88 μg L−1 respectively. C. vulgaris spiked with increasing concentration of metal solutions showed the expected trend of increased Cu, Cd and Pb contents in the medium after exposure for 72 hours (Fig. 3A–C, column plots). C. vulgaris Cu uptake capacity decreased from 70% to 15% when exposed to 10, 50 and 100 μM of CuCl2 respectively. Interestingly, the presence of high CuCl2 (200 and 500 μM) in the medium improved the Cu uptake capacity. A similar metal uptake trend was observed for Cd-spiked cultures. In contrast, Pb-spiked cultures showed consistently high uptake capacity (75% to 85%) (Fig. 3A–C, line plot).Elemental analysis of algal biomass showed that the accumulation of Cu, Cd and Pb by C. vulgaris were parallel to increasing metal concentrations in the culture medium (Fig. 3D–F, column plot). This demonstrated that the metals removed from the medium were indeed uptaken and bioaccumulated in the biomass. The line plot in Fig. 3D–F also showed that all metals were more effectively bioaccumulated by C. vulgaris at higher metal concentrations. A comparison of the biomass metal content revealed that C. vulgaris accumulated a significantly larger amount of Pb than Cu and Cd, in the order of Pb2+ > Cd2+ > Cu2+. This showed that metal uptake from the surrounding medium is greatly influenced by the metal species and concentrations, which is in agreement with previous studies.10,54
While the uptake of high concentrations of heavy metals is a rare phenomenon in plants, a particular plant group known as the hyperaccumulating or metal-tolerant species has been widely reported to accumulate notable amounts of essential and non-essential metals in their leaves and roots. Vascular plants such as Silene vulgaris, Thlaspi caerulescens, Alyssum lesbiacum, Arabidopsis halleri and Brassica spp.4,5 are some model species for understanding the metal accumulation and tolerance mechanisms, with the ultimate aim of utilizing these species for phytoremediation. Several studies had shed light on the metal uptake and accumulation mechanisms in these hyperaccumulating plant species, which involved (1) passive absorption of metals to charged polysaccharides in the cell wall and intracellular matrix; (2) active metal uptake against large intracellular concentration gradients.10,55,56 Metals bound on the cell wall are transported across the plasma membrane, and the driving force of metal uptake is the presence of free chelating molecules in the algal cytoplasm.
Phytochelatins is a common class of metal chelating molecules responsible for the binding to free metal ions. Based on a pioneering study by Grill and coworkers,57 the production of phytochelatins is catalyzed by phytochelatin synthase (PC synthase), where the activation of PC synthase is dependent on the type and concentration of free metal ions. It is reported that a high intracellular metal content is more efficient in activating PC synthase, thus inducing the production of more PCs. According to their study on PC synthase activity, Cd2+ was determined as the best metal activator followed by Pb2+ and Cu2+. This coincided with our observations that greater amount of metal ions were accumulated in the biomass when the cultures are exposed to higher metal concentrations. The amount of Cd (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1 dried biomass) accumulated in the biomass was also significantly higher than Cu (8000 mg kg−1 dried biomass). However, the exceptionally high Pb content in C. vulgaris (80
000 mg kg−1 dried biomass) accumulated in the biomass was also significantly higher than Cu (8000 mg kg−1 dried biomass). However, the exceptionally high Pb content in C. vulgaris (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1 dried mass) which did not follow the PC synthase metal activation trend, may suggest that Pb-detoxification was not attributed to PC chelation. As discussed by Afkar and coworkers,10 heavy metals can be uptaken by algae both passively and actively. While Pb favors passive adsorption to the algal cell wall, Cd and Cu are actively accumulated in the algal cells by phytochelatins. With support from these studies, we suggest that the conflicting trend of metal accumulation in the order of Pb2+ > Cd2+ > Cu2+, with PC synthase activation by Cd2+ > Pb2+ > Cu2+ was due to metal-specific uptake mechanisms by C. vulgaris. Chelating molecules, phytochelatins and glutathione, were subsequently quantified to further reveal whether chelating molecules influence metal uptake capacity.
000 mg kg−1 dried mass) which did not follow the PC synthase metal activation trend, may suggest that Pb-detoxification was not attributed to PC chelation. As discussed by Afkar and coworkers,10 heavy metals can be uptaken by algae both passively and actively. While Pb favors passive adsorption to the algal cell wall, Cd and Cu are actively accumulated in the algal cells by phytochelatins. With support from these studies, we suggest that the conflicting trend of metal accumulation in the order of Pb2+ > Cd2+ > Cu2+, with PC synthase activation by Cd2+ > Pb2+ > Cu2+ was due to metal-specific uptake mechanisms by C. vulgaris. Chelating molecules, phytochelatins and glutathione, were subsequently quantified to further reveal whether chelating molecules influence metal uptake capacity.
Quantification of glutathione and phytochelatins
Phytochelatins (PCs) were analyzed as these classes of compounds were potential Cu and Cd chelators in C. vulgaris. Moreover, the production of PCs was solely induced in the presence of excess metal ions, and no other environmental factors were known to influence PC accumulation; thus the amount of PCs present in C. vulgaris could be related to specific metal detoxification response.58 Glutathione (GSH), a multi-functional molecule was also analyzed as it plays a significant role in maintaining redox homeostasis in metal-exposed plant cells by efficient removal or metabolism of ROS via the ascorbate–glutathione pathway.58 In addition, GSH is the sole precursor for PC biosynthesis of plant cells exposed to heavy metals.1,59 The proposed metabolic pathways of metal detoxification focusing on the roles of GSH, GSSG and PCs are illustrated in Fig. S7 (ESI†). The limits of quantification of GSH, GSSG, PC2 and PC3 were 75.21 μg L−1, 132.16 μg L−1, 8.65 μg L−1 and 8.00 μg L−1 respectively. Based on Table 2, two individual trends of GSH were observed for redox active Cu and non-redox active heavy metals (Cd and Pb). The GSH content decreased with increasing Cu concentrations. However, increasing GSH contents were observed when C. vulgaris was exposed to higher Cd and Pb concentrations. Changes in the GSH level could be a consequence of GSH oxidation to GSSG, to mediate the enhanced ROS generation in plant cells subjected to heavy metals, or utilized as precursors for the PC biosynthesis.58| Metal treatments | μM | GSH | GSH/GSSG ratio | PC2 | PC3 | |||
|---|---|---|---|---|---|---|---|---|
| Mean | Std dev | Mean | Std dev | Mean | Std dev | |||
| Copper-spiked samples | 0 | 310.47 | 26.32 | 0.777 | 1.96 | 0.11 | ND | — |
| 10 | 305.99 | 26.08 | 0.647 | 6.78 | 1.15 | ND | — | |
| 50 | 384.02 | 28.94 | 0.618 | 29.51 | 3.68 | 6.93 | 1.10 | |
| 100 | 376.77 | 5.77 | 0.759 | 42.25*** | 1.78 | 14.43*** | 1.01 | |
| 200 | 85.68*** | 6.59 | 0.435 | 42.62*** | 6.51 | 7.84 | 0.54 | |
| 500 | 150.43*** | 10.28 | 0.698 | 77.62*** | 3.29 | 15.93*** | 1.79 | |
| Cadmium-spiked samples | 0 | 479.67 | 39.38 | 1.097 | ND | — | ND | — |
| 10 | 481.40 | 29.78 | 0.911 | 3.52 | 0.25 | ND | — | |
| 50 | 499.70 | 34.24 | 1.195 | 5.77 | 0.50 | 9.52 | 0.25 | |
| 100 | 437.22 | 22.92 | 2.038 | 71.81*** | 7.86 | 32.87*** | 4.89 | |
| 200 | 622.65*** | 35.52 | 1.649 | 223.24*** | 10.60 | 201.19*** | 18.46 | |
| 500 | 616.44*** | 11.74 | 4.861 | 405.05*** | 12.07 | 953.88*** | 32.46 | |
| Lead-spiked samples | 0 | 449.65 | 32.24 | 1.163 | ND | — | ND | — |
| 10 | 468.82 | 27.05 | 1.538 | ND | — | ND | — | |
| 50 | 453.80 | 30.05 | 1.402 | ND | — | ND | — | |
| 100 | 495.23 | 16.41 | 1.239 | ND | — | ND | — | |
| 200 | 486.95 | 18.33 | 1.240 | ND | — | ND | — | |
| 500 | 652.69*** | 29.46 | 1.781 | ND | — | ND | — | |
The depletion of GSH in Cu-spiked cultures suggested rapid consumption of GSH for PC synthesis, and/or oxidation of GSH to GSSG. The decreased GSH levels with a significant increment of PC2 and PC3 when exposed to greater than 50 μM CuCl2 confirmed that GSH was utilized for PC production. The significant accumulation of Cu in algal biomass (Fig. 3D) spiked with greater than 50 μM CuCl2 also supported that high Cu concentrations induced the production of PC2 and PC3, and these PCs were involved in the accumulation of Cu in C. vulgaris. Many studies have suggested the use of the GSH/GSSG ratio as an indicator of cellular redox imbalance, where a low GSH/GSSG ratio indicates oxidative stress.60,61 A comparison of the GSH/GSSG ratios in various metal-spiked cultures showed that redox active Cu2+ is more potent in inducing oxidative stress than non-redox active Cd2+ and Pb2+. This is in agreement with our metabolomics study where organic osmolytes (GPC and betaine) were significantly reduced in C. vulgaris spiked with greater than 200 μM CuCl2, but no significant changes were observed for Cd and Pb-spiked cultures. Growth inhibition of C. vulgaris spiked with 200 μM and 500 μM CuCl2 respectively (Fig. S1, ESI†) further revealed that C. vulgaris was unable to cope with the increasing Cu load, leading to higher oxidative stress. Interestingly, C. vulgaris spiked with 500 μM CuCl2 has a higher GSH and PC content than the 200 μM CuCl2-spiked culture. This suggests that C. vulgaris undergoes metal homeostasis rather than metal toxicity at higher Cu concentrations. The improved GSH/GSSG ratio for 500 μM CuCl2-spiked cultures also revealed C. vulgaris ability to regulate metal toxicity. Therefore, we agree that metal-induced oxidative stress is typically related to the metal species and metal concentration exposed to the plant cells.58
The substantial increment of PC2 and PC3 in Cd-spiked C. vulgaris suggested that PC biosynthesis was activated in the presence of more than 100 μM CdCl2. The concentration-dependent accumulation of Cd observed in Fig. 3E was in parallel with the PC production, which further revealed a relationship between PC availability and Cd accumulation. Although a higher concentration of Cd2+ was found in C. vulgaris as compared to Cu2+, the high GSH/GSSG ratio for Cd-spiked cultures indicated lower Cd-induced oxidative stress. The lower Cd-induced oxidative stress could be a result of more GSH, PC2 and PC3 produced with increasing Cd concentrations. The thiol compounds (GSH, PC2 and PC3) could reduce metal availability by high affinity binding to free metal ions, which would otherwise induce direct or indirect ROS production, leading to oxidative stress.14 Our observed trends were in line with previous studies on marine and freshwater algae, which demonstrated that the total PC content increased with increasing Cu and Cd concentrations.62,63 However, the expected PC accumulation induced by Pb was not observed in C. vulgaris. The undetectable PC levels despite a significantly high Pb content as determined in our elemental analysis indicated the possibility of a different Pb accumulation mechanism. The relatively consistent GSH/GSSG ratio revealed that C. vulgaris was not experiencing significant oxidative stress, regardless of the Pb exposure concentrations. This suggests that the excess Pb present in C. vulgaris was either detoxified by other metal chelators or passively adsorbed onto the cell wall rather than actively uptaken into the cell cytoplasm. This latter opinion was in agreement with Afkar and coworkers,10 where Pb favours absorption to the charged polysaccharides on the algal cell wall, over active uptake into the cytoplasm. Some studies also revealed that PC synthesis is not the only driving force for metal bioaccumulation, especially for hyperaccumulators.64,65 Apart from PCs, GSH was also reported to facilitate metal detoxification, with studies demonstrating the accumulation of GSH concentrations in hyperaccumulating plants.66 Although PCs were generally not detected in Pb-spiked cultures, an increased GSH level was observed at high Pb concentrations. Therefore, we proposed that the major Pb removal mechanism is via biosorption to C. vulgaris cell wall, and excess free Pb2+ that were actively uptaken into the cytoplasm were possibly detoxified by GSH chelation.
Conclusion
In this study, we took on a metabolomics approach to shed light on the metabolic responses of green microalgae as a consequence of metal stress. We established a comprehensive study on the effect of different metal species and metal concentrations on C. vulgaris using a range of analytical techniques. With the application of NMR-based metabolomics and multivariate data analysis, metabolites contributing to significant metabolic changes in C. vulgaris exposed to different concentrations of CuCl2, CdCl2 and Pb(NO3)2 were simultaneously determined. Substantial decrease in the sucrose content and some major amino acids revealed that C. vulgaris was experiencing photosynthesis impairment in a high Cu environment. Decreased organic osmolyte levels in Cu-spiked cultures also indicated Cu-induced oxidative stress, which were in agreement with the lower GSH/GSSG ratio determined by the LC-MS/MS analysis. A clear relationship between Cu toxicity and redox homeostasis in green microalgae was revealed; high Cu concentrations led to redox imbalance in C. vulgaris. As C. vulgaris failed to achieve redox homeostasis, its growth and development was substantially affected. Growth inhibition and photosynthesis impairment confirmed that exposure to high Cu concentrations after 72 h was toxic to C. vulgaris. However, these metabolic responses were not observed in Cd and Pb-spiked cultures, which revealed that redox active Cu2+ is more potent than non-redox active Cd2+ and Pb2+ in inducing redox imbalance. In contrast, elevated levels of alanine and acetate were found in C. vulgaris spiked with high Cd and Pb concentrations. With reference from previous studies that alanine accumulation is a consequence of hypoxia, we suggest for the first time the role of metal-induced hypoxic stress. However, further studies need to be carried out to establish the relationship between metal exposure and oxygen availability in C. vulgaris cultures. Additionally, the ICP-MS and LC-MS/MS analyses revealed a linear relationship between Cu and Cd exposure, and GSH and PC availability in algal biomass. This demonstrated the activation of C. vulgaris metal detoxification mechanisms which include the removal of free Cu2+ and Cd2+via PC-chelation, and metabolism of ROS via the glutathione–ascorbate cycle. The higher PC and GSH contents, and lower GSH/GSSG ratio in Cd-spiked cultures as compared to Cu-spiked cultures, further revealed that C. vulgaris was able to achieve redox homeostasis in high Cd environment due to its efficient Cd detoxification mechanism. Lastly, the accumulation of Pb was not attributed to PCs as shown by the absence of PCs in algal biomass, but rather possibly due to biosorption onto algal cell walls and interaction with GSH.Acknowledgements
The authors would like to thank all of the people from NUS Environmental Research Institute (NERI) and the NERI-Agilent Research Alliance who provided valuable advice and support in our research. This publication would not have been possible without their assistance. Special thanks to Elaine Tay and Karen Laserna for their editorial assistance. Lastly, we would like to acknowledge financial support from the National University of Singapore, National Research Foundation and Economic Development Board (SPORE, COY-15-EWI-RCFSA/N197-1), Ministry of Education (R-143-000-519-112), and Shenzhen Development and Reform Commission (SZ DRC).Notes and references
- S. Clemens, Biochimie, 2006, 88, 1707–1719 CrossRef CAS PubMed
.
- S. M. Gallego, L. B. Pena, R. A. Barcia, C. E. Azpilicueta, M. F. Iannone, E. P. Rosales, M. S. Zawoznik, M. D. Groppa and M. P. Benavides, Environ. Exp. Bot., 2012, 83, 33–46 CrossRef CAS PubMed
.
- A. D. Peuke and H. Rennenberg, EMBO Rep., 2005, 6, 497–501 CrossRef CAS PubMed
.
- S. Clemens, M. G. Palmgren and U. Krämer, Trends Plant Sci., 2002, 7, 309–315 CrossRef CAS
.
- U. Krämer, New Phytol., 2003, 158, 4–6 CrossRef
.
- M. A. Hubbe, S. H. Hasan and J. J. Ducoste, BioResources, 2011, 6, 2161–2286 Search PubMed
.
- S. K. Mehta and J. P. Gaur, Crit. Rev. Biotechnol., 2005, 25, 113–152 CrossRef CAS PubMed
.
- F. A. Abu Al-Rub, M. H. El-Naas, F. Benyahia and I. Ashour, Process Biochem., 2004, 39, 1767–1773 CrossRef CAS PubMed
.
- B. Volesky and Z. R. Holan, Biotechnol. Prog., 1995, 11, 235–250 CrossRef CAS PubMed
.
- E. Afkar, H. Ababna and A. A. Fathi, Am. J. Environ. Sci., 2010, 6, 230–237 CrossRef CAS
.
- S. Carfagna, N. Lanza, G. Salbitani, A. Basile, S. Sorbo and V. Vona, Springerplus, 2013, 2, 147 CrossRef PubMed
.
- W. B. Dunn, N. J. Bailey and H. E. Johnson, Analyst, 2005, 130, 606–625 RSC
.
- E. Holmes, H. Tang, Y. Wang and C. Seger, Planta Med., 2007, 72, 771–785 CrossRef PubMed
.
- S. S. Sharma and K.-J. Dietz, Trends Plant Sci., 2009, 14, 43–50 CrossRef CAS PubMed
.
- P. Mohanpuria, N. K. Rana and S. K. Yadav, Environ. Toxicol., 2007, 22, 368–374 CrossRef CAS PubMed
.
- P. Sharma and R. S. Dubey, Braz. J. Plant Physiol., 2005, 17, 35–52 CrossRef CAS
.
- S. K. Yadav, S. Afr. J. Bot., 2010, 76, 167–179 CrossRef CAS PubMed
.
- A. M. Reddy, S. G. Kumar, G. Jyonthsnakumari, S. Thimmanaik and C. Sudhakar, Chemosphere, 2005, 60, 97–104 CrossRef CAS PubMed
.
- E. R. Stadtman and C. N. Oliver, J. Biol. Chem., 1991, 266, 2005–2008 CAS
.
- H. C. Bold, Bull. Torrey Bot. Club, 1949, 76, 101–108 CrossRef
.
- H. W. Bischoff and H. C. Bold, Univ. Tex. Publ., 1963, 6318, 1–95 Search PubMed
.
- J. Xia, R. Mandal, I. Sinelnikov, D. Broadhurst and D. S. Wishart, Nucleic Acids Res., 2012, 40, W127–W133 CrossRef CAS PubMed
.
- J. Xia, N. Psychogios, N. Young and D. S. Wishart, Nucleic Acids Res., 2009, 37, W652–W660 CrossRef CAS PubMed
.
- Q. Cui, I. A. Lewis, A. D. Hegeman, M. E. Anderson, J. Li, C. F. Schulte, W. M. Westler, H. R. Eghbalnia, M. R. Sussman and J. L. Markley, Nat. Biotechnol., 2008, 26, 162 CrossRef CAS PubMed
.
-
C. T. Chiou, Partition and Adsorption of Organic Contaminants in Environmental Systems, John Wiley & Sons, Inc., Hoboken, NJ, 2002, p. 257 Search PubMed
.
- D. B. D. Simmons, A. R. Hayward, T. C. Hutchinson and R. J. N. Emery, Anal.
Bioanal. Chem., 2009, 395, 809–817 CrossRef CAS PubMed
.
- M. Minocha, P. Thangavel, O. P. Dhankher and S. Long, J. Chromatogr., A, 2008, 1207, 72–83 CrossRef PubMed
.
-
K.-J. Dietz, U. Krämer and M. Baier, Heavy metal stress in plants: Molecules to ecosystem, Springer, 1999, pp. 73–97 Search PubMed
.
- N. M. Franklin, J. L. Stauber, R. P. Lim and P. Petocz, Environ. Toxicol. Chem., 2002, 21, 2412–2422 CAS
.
- A. Bajguz, Arch. Environ. Contam. Toxicol., 2011, 60, 406–416 CrossRef CAS PubMed
.
- M. R. Clausen, V. Bach, M. Edelenbos and H. C. Bertram, J. Agric. Food Chem., 2012, 60, 9495–9501 CrossRef CAS PubMed
.
- L. Webershirch, A. Luna, S. Skoglund and H. This, Anal. Bioanal. Chem., 2011, 399, 483–487 CrossRef PubMed
.
- M. S. Chauton, T. R. Størseth and G. Johnsen, J. Appl. Phycol., 2003, 15, 533–542 CrossRef
.
- M. Liebeke and J. G. Bundy, Biochem. Biophys. Res. Commun., 2013, 430, 1306–1311 CrossRef CAS PubMed
.
- V. Gupta, R. S. Thakur, C. R. K. Reddy and B. Jhaa, RSC Adv., 2013, 3, 7037–7047 RSC
.
- A. Meister, J. Biol. Chem., 1988, 263, 205–208 Search PubMed
.
- M. B. Burg, J. D. Ferraris and N. I. Dmitrieva, Physiol. Rev., 2007, 87, 1441–1474 CrossRef CAS PubMed
.
- M. Ashraf and M. R. Foolad, Environ. Exp. Bot., 2007, 59, 206–216 CrossRef CAS PubMed
.
- V. Vasiliou, A. Bairoch, K. F. Tipton and D. W. Nebert, Pharmacogenetics, 1999, 9, 421–434 CrossRef CAS PubMed
.
- M. Gallazzini, J. D. Ferraris, M. Kunin, R. G. Morris and M. B. Burg, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15260–15265 CrossRef CAS PubMed
.
- M. Hiraoka, A. Abe and J. A. Shayman, J. Lipid Res., 2005, 46, 2441–2447 CrossRef CAS PubMed
.
- J. A. Rosas-Rodríguez and E. M. Valenzuela-Soto, Life Sci., 2010, 87, 515–520 CrossRef PubMed
.
- N. Ratkevicius, J. A. Correa and A. Moenne, Plant, Cell Environ., 2003, 26, 1599–1608 CrossRef CAS
.
- N. Mallick, J. Plant Physiol., 2004, 161, 591–597 CrossRef CAS PubMed
.
- S. R. Devi and M. N. V. Prasad, Russ. J. Plant Physiol., 2005, 52, 205–208 CrossRef CAS PubMed
.
- B. N. Tripathi, S. K. Mehta, A. Amar and J. P. Gaur, Chemosphere, 2006, 62, 538–544 CrossRef CAS PubMed
.
- A. M. Limami, G. Glévarec, C. Ricoult, J.-B. Cliquet and E. Planchet, J. Exp. Bot., 2008, 9, 2325–2335 CrossRef PubMed
.
- C. Catalanotti, W. Yang, M. C. Posewitz and A. R. Grossman, Front. Plant Sci., 2013, 4, 1–17 CrossRef PubMed
.
- M. M. Sachs, C. C. Subbaiah and I. N. Saab, J. Exp. Bot., 1996, 47, 1–15 CrossRef CAS
.
- M. Rocha, F. Licausi, W. L. Araújo, A. Nunes-Nesi, L. Sodek, A. R. Fernie and J. T. van Dongen, Plant Physiol., 2010, 152, 1501–1513 CrossRef CAS PubMed
.
- Y. Miyashita, R. Dolferus, K. P. Ismond and A. G. Good, Plant J., 2007, 49, 1108–1121 CrossRef CAS PubMed
.
- C. A. F. de Sousa CAF and L. Sodek, Environ. Exp. Bot., 2003, 50, 1–8 CrossRef
.
- S. Wang, D. Zhang and X. Pan, Chemosphere, 2013, 93, 230–237 CrossRef CAS PubMed
.
- A. A. Fathi, F. T. Zaki and H. A. Ibraheim, Protistology, 2005, 4, 73–78 CAS
.
- S. Clemens, Planta, 2001, 212, 475–486 CrossRef CAS
.
- J. L. Halls, J. Exp. Bot., 2002, 53, 1–11 CrossRef
.
- E. Grill, S. Löffler, E. L. Winnacker and M. H. Zenk, Proc. Natl. Acad. Sci. U. S. A., 1989, 86, 6838–6842 CrossRef CAS
.
- N. A. Anjum, I. Ahmad, I. Mohmood, M. Pacheco, A. C. Duarte, E. Pereira, S. Umar, A. Ahmad, N. A. Khan, M. Iqbal and M. N. V. Prasad, Environ. Exp. Bot., 2012, 75, 307–324 CAS
.
-
S. Srivalli and R. Khanna-Chopra, Sulfur assimilation and abiotic stress in plants, Springer-Verlag, Berlin/Heidelberg, 2008, pp. 207–225 Search PubMed
.
- A. H. Millar, V. Mittova and G. Kiddle, Plant Physiol., 2003, 133, 443–447 CrossRef CAS PubMed
.
- C. H. Foyer and G. Noctor, Plant, Cell Environ., 2005, 28, 1056–1071 CrossRef CAS
.
- E. Morelli and G. Scarano, Mar. Environ. Res., 2001, 52, 383–395 CrossRef CAS
.
- A. Bajguz, Arch. Environ. Contam. Toxicol., 2011, 60, 406–416 CrossRef CAS PubMed
.
- S. Ebbs, I. Lau, B. Ahner and L. Kochian, Planta, 2002, 214, 635–640 CrossRef CAS
.
- J. Hernandez-Allica, C. Garbisu, J. M. Becerril, O. Barrutia, J. L. García-Plazaola, F. J. Zhao and S. P. Mcgrath, Plant, Cell Environ., 2006, 29, 1422–1429 CrossRef CAS
.
- Q. Sun, Z. H. Ye, X. R. Wang and M. H. Wong, J. Plant Physiol., 2007, 164, 1489–1498 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Additional multivariate data analysis projections and metabolic pathways. See DOI: 10.1039/c3mb70425d |
| This journal is © The Royal Society of Chemistry 2014 |