Biomarkers of primary dysmenorrhea and herbal formula intervention: an exploratory metabonomics study of blood plasma and urine†
Pei Liua, Jinao Duan*a, Peijuan Wangb, Dawei Qiana, Jianming Guoa, Erxin Shanga, Shulan Sua, Yuping Tanga and Zongxiang Tanga
aJiangsu Key Laboratory for High Technology Research of TCM Formulae, Nanjing University of Chinese Medicine, Nanjing 210046, PR China. E-mail: duanja@163.com; Fax: +86-25-85811116; Tel: +86-25-85811116
bDepartment of Obstetrics and Gynecology, Jiangsu Province Hospital on Integration of Chinese and western Medicine, Nanjing 210028, PR China
First published on 12th October 2012
Abstract
Primary dysmenorrhea (PDM), a common clinical endocrine disorder affecting young women, is associated with endocrinopathy and metabolic abnormalities. Although some physiological and pathological function parameters have been investigated, little information about the changes of small metabolites in biofluids has been reported, which may cause poor diagnosis and treatment for PDM. The Xiang-Fu–Si-Wu Formula (XFSWF) is a Chinese herbal formula used to treat PDM for hundreds of years. The aim of this study was to establish the metabolic profile of PDM and investigate the action mechanism of XFSWF effect. In this cross-sectional study of 25 patients with PDM and 12 healthy controls, contents of small molecular endogenous metabolites in blood plasma and urine samples were measured by ultra performance liquid chromatography (UPLC) coupled with quadrupole-time-of-flight mass spectrometry (QTOF/MS) and triple quadrupole mass spectrometry (QqQ/MS) based techniques and analyzed by multivariate statistical methods. The levels of LPCs including lypso (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), lysoPC(20
1), lysoPC(20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), lysoPC(18
4), lysoPC(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), lysoPC(16
2), lysoPC(16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), lysoPC(18
0), lysoPC(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), lysoPC(10
1), lysoPC(10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), estrone, 17-hydroxyprogesterone, myristoylglycine and palmitoylglycine increased significantly (p < 0.05) in PDM, while the levels of phytosphingosine, dihydrocortisol and sphingosine decreased significantly (p < 0.05) compared with the healthy controls. These significant perturbations are involved in glycerophospholipid metabolism and sphingolipid metabolism, as well as steroid hormone biosynthesis. The metabolic deviations recovered to the normal level after XFSWF intervention. The results demonstrated that biofluids metabonomics was a powerful tool in clinical diagnosis and treatment of PDM for providing information on changes in metabolites and neural, endocrinal and immune pathways. XFSWF can be used for the treatment of PDM cases, especially for those adolescents who do not desire a contraceptive method, to reduce the risk of secondary dysmenorrhea.
1), estrone, 17-hydroxyprogesterone, myristoylglycine and palmitoylglycine increased significantly (p < 0.05) in PDM, while the levels of phytosphingosine, dihydrocortisol and sphingosine decreased significantly (p < 0.05) compared with the healthy controls. These significant perturbations are involved in glycerophospholipid metabolism and sphingolipid metabolism, as well as steroid hormone biosynthesis. The metabolic deviations recovered to the normal level after XFSWF intervention. The results demonstrated that biofluids metabonomics was a powerful tool in clinical diagnosis and treatment of PDM for providing information on changes in metabolites and neural, endocrinal and immune pathways. XFSWF can be used for the treatment of PDM cases, especially for those adolescents who do not desire a contraceptive method, to reduce the risk of secondary dysmenorrhea.
Introduction
Primary dysmenorrhea (PDM, menstrual pain without pelvic abnormality) is one of the most common gynaecological disorders in young women.1 PDM affects almost half of women in the world, with symptoms becoming severe in 10% of them so as to disrupt their routine activities.2 In China, 33.2% women suffer from dysmenorrhea and 36.1% of dysmenorrheal patients from PDM. The morbidity of PDM among Chinese female undergraduate students is from 60–80%.3–4Although PDM is not fatal and causes no organ failure, whereas secondary dysmenorrhea (SDM) is a symptom of uterine abnormalities or adnexal diseases, it may affect the patient’s life and result in disability and inefficiency. PDM may cause complicated social, economic and familial problems, including disruption of routine activities, absence from school or workplace, medication abuse and physical and mental disorders, opinion change of femininity and even the method of childbirth chosen.5 In USA, an annual loss of 600 million hours and US$2 billion is attributable to dysmenorrhea.6
The etiology of primary dysmenorrhea is not precisely understood, but most symptoms can be explained by the action of uterine prostaglandins. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most common pharmacologic treatments for PDM. These drugs are useful due to their ability to decrease the production of prostaglandins. Several other hormonal methods exist for treating PDM, such as oral contraceptive pills, Depo-Provera (a branded progestogen – only contraceptive, depot medroxyprogesterone acetate (DMPA)) and Mirena (Levonorgestrel intrauterine device). However, about 10% women affected by PDM do not respond to these measures.7 NSAIDs are also intolerable and have many side effects on the liver, kidney and digestive function. A hormonal device has side effects such as nausea and water retention.8–10
Due to the shortcomings of NSAIDs and hormonal methods, Chinese medicinal therapy is considered as a feasible alternative in the treatment of PDM.11 In Traditional Chinese Medicine (TCM), PDM is divided into different disharmony patterns (may be subtypes of disease in Western Medicine) such as syndrome of Qi stagnation and blood stasis. It is considered that pressure from life and work makes women stressed or causes depression and finally results in PDM. One herbal formula named Xiang-Fu–Si-Wu Formula (XFSWF) has been used as the typical treatment for PDM with Qi stagnation and blood stasis syndrome since 1880 AD in China. In modern clinical efficacy evaluation, the total clinical effectiveness of XFSWF for treating PDM is 80–90%.12–14 Moreover, our study has shown that XFSWF inhibits COX-2 activity, decreases the 5-HIAA level in hypothalamus of dysmenorrheal syndrome rats, decreases the Ca2+ level and increases the NO level in uterus homogenates of mice and rats with dysmenorrhea syndrome.15–17 The effective constituents of XFSWF are numerous and diverse, including phthalide, organic acids, alkaloids, etc.18
Until now, the pathogenesis of PDM is elusive. The neuro-endocrine-immune (NEI) system, such as prostaglandins, ovarian hormones, cervical factors, vasopressin, nerves and psychological factors, was considered to be possible important markers in the pathology of PDM.19,20 Classical TCM therapy on PDM syndrome also considered the molecular basis with the NEI system.21,22 Traditional markers detected by conventional clinical chemistry and histopathology methods were not region specific and fraught with difficultly. The lack of a systematic molecular diagnosis for PDM means its therapies are poorly targeted and could be ineffective.
Metabonomics is a research method for the analysis of low molecular weight compounds to systematic investigation of metabolic responses in biological systems to genetic or environmental stimuli.23 There has been much research on the metabonomics of disease, especially in cancers and metabolic diseases.24,25
The profile of the metabolome is closely correlated with traditional biological and clinical end points. Metabolite changes have been observed in diseased individuals as a primary indicator.26 The global qualitative and quantitative analysis of small molecules present in biofluids and tissues shows great promise as a means to identify biomarkers of drug efficacy.27–29
Various analytical methods are available for metabonomic research, among which mass spectrometry (MS) is favored because of its wide dynamic range, reproducible quantitative analysis, and the ability to analyze biofluids with extreme molecular complexity.30 Although none of the existing analysis techniques could analyze all the compounds in the metabolic group,31,32 metabonomics based on MS is a powerful technique that enables the parallel assessment of the levels of a broad range of metabolites and has a great impact in the investigation of physiological status, diagnosing diseases and identifying perturbed pathways due to disease or drug treatment.33
To our knowledge, there have been no previous reports of metabolic profiling studies on PDM biofluids and metabolic alterations with XFSWF intervention. In this study, by using MS-based metabonomic techniques, we present a plasma and urine metabolic profiling study on PDM women having Qi stagnation syndrome to predict and beneficial effects of XFSWF intervention. All of the clinical studies described have been carried out on female college students in China whose average age is 20–28 after strict selection. This is the first PDM metabolic profiling study in Chinese medicine suggesting that plasma and urine testing for PDM is a tangible possibility to clarify metabolic alterations with PDM subtype and TCM intervention.
Experimental
Study design
Plasma and urine samples were collected from patients in Nanjing University of Chinese Medicine. Ethical approval was granted by the research ethics committees at Jiangsu Province Hospital on Integration of Chinese and Western Medicine. After the clinical trial had been clearly explained to the patients, written consent was obtained from all subjects.The patients had no organic lesion of the reproductive system, but had premenstrual or menstrual distending pain in lower abdomen for at least three continuous menstrual cycles. Visual analogue scale (VAS, a bar with 0–10 cm: 0 cm representing no pain, 10 cm, severe pain) was adopted to evaluate pain degree.34,35 The pain criteria for inclusion was more than 4 (Table S1, ESI†). The Qi stagnation syndrome of PDM was diagnosed by mammary swelling pain, thoracic discomfort, dark purple tongue and wiry pulse; and twelve healthy female students were recruited as healthy control subjects. A detailed medical questionnaire was completed to ensure that subjects were eligible for the study (Text 1S, ESI†).
In this cross-sectional study, a total of forty-three dysmenorrheal patients were assessed for eligibility; in the enrollment stage, five patients were diagnosed to be having secondary dysmenorrhea (SDM) such as endometriosis and hysteromyoma. Nine patients did not meet the TCM syndrome evaluation criteria. Four patients refused to participate in the study.
The remaining twenty-five patients were given the therapeutic treatment. Participants were observed for 4 menstrual cycles: they were treated with XFSWF for 3 menstrual cycles and follow-up for 1 menstrual cycle. The therapeutic effect was comprehensively measured by the primary outcome measure (VAS) and the secondary outcome measure (TCM syndrome evaluation). The outcomes were obtained and compared using a self-control method.
Subject demographics are shown in Table 1. The median age, college education, weight, body-mass index (BMI) and menstrual cycle length mean of the PDM group were not significantly higher than the healthy controls (p > 0.05).
| Characteristics | Healthy controls | PDM | p-Value |
|---|---|---|---|
| n | 12 | 25 | |
| Age mean (SD), years | 24.2 (1.6) | 24.1 (1.9) | 0.97 |
| College education, n (%) | 12 (100) | 21 (100) | 1.00 |
| Weight mean (SD), kg | 49.3 (2.1) | 48.6 (2.3) | 0.39 |
| Body-mass index mean (SD), kg m−2 | 19.3 (0.5) | 18.9 (0.9) | 0.07 |
| Menstruation duration mean (SD), days | 5.4 (0.8) | 5.0 (0.9) | 0.25 |
| Menstrual cycle length mean (SD), days | 29.9 (0.8) | 31.0 (2.3) | 0.06 |
Sample preparation
XFSWF dispensing granule produced in Jiangyin Tianjiang Pharmaceutical Co., Ltd was used as therapeutic drug in this study.One dosage of XFSWF was composed of Angelicae sinensis Radix (9 g), Chuanxiong Rhizoma (4.5 g), Paeoniae Radix Alba (4.5 g), Rehmanniae Radix Praeparata (12 g), Cyperi Rhizoma (4.5 g), Aucklandiae Radix (3 g) and Corydalis Rhizoma (4.5 g). The mixture herbals were refluxed with 10 times water for 2 times. The filtrates were combined and concentrated below 70 °C to obtain a certain volume at the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w, weight of all constituting herbs and the extract filtrates) under vacuum. 95% ethanol was added to the extract filtrates until the concentration of ethanol was adjusted to 80%. Ethanol was removed below 70 °C under vacuum to obtain 2 g XFSWF extract. Dextrin (8 g) was added to the extract. By a sprayed-drying process,36 A XFSWF dispensing granule was obtained.
1 (w/w, weight of all constituting herbs and the extract filtrates) under vacuum. 95% ethanol was added to the extract filtrates until the concentration of ethanol was adjusted to 80%. Ethanol was removed below 70 °C under vacuum to obtain 2 g XFSWF extract. Dextrin (8 g) was added to the extract. By a sprayed-drying process,36 A XFSWF dispensing granule was obtained.
The method of UPLC-DAD-ESI-MS was adopted to screen and analyze the potential active components in the XFSWF extract. Compounds including aromatic acid, phthalide, alkaloid, and terpenes etc. were identified. Quantitative analysis of main targeted compounds was achieved using MS detection. Twenty peaks were indicated on the UPLC-MS fingerprint chromatograph of the XFSWF extract. The relative retention time and relative peak area were obtained with chloromycetin (negative mode) and clarithromycin (positive mode) as internal standard. The area of unknown peaks was no more than 30% of total peak areas. The content of gallic acid (6.68 mg g−1 dry extract), caffeic acid (1.72 mg g−1 dry extract), chlorogenic acid (8.13 mg g−1 dry extract), albiflorin (54.4 mg g−1 dry extract), peoniflorin (68.7 mg g−1 dry extract), ferulaic acid (6.91 mg g−1 dry extract), tetrahydrocolumbamine (0.94 mg g−1 dry extract), protopine (1.63 mg g−1 dry extract), tetrahydrocoptisine (0.83 mg g−1 dry extract), tetrahydropalmatine (1.54 mg g−1 dry extract), corydaline (7.92 mg g−1 dry extract), berberine (0.82 mg g−1 dry extract) and dehydrocorydaline (8.64 mg g−1 dry extract) was determined by reference compounds. The content of neolazppaic acid (3.22 mg g−1 dry extract), 3-butylidene-7-hydroxyphthalide (12.5 mg g−1 dry extract), costunolide (3.02 mg g−1 dry extract), palmitic acid (2.40 mg g−1 dry extract), allocryptopine (0.72 mg g−1 dry extract), glaucine (0.73 mg g−1 dry extract) and costuslactone (0.63 mg g−1 dry extract) was determined by relative content to internal standard. (Fig. S1, Table S2, ESI†).
The XFSWF dispensing granule at a dosage (10 g) of 200 mL was taken twice a day in the morning and afternoon. First dosage of XFSWF was taken from the first day of a menstrual cycle, with successive application for 5 days. The next two menstrual cycles, XFSWF was taken from 5 days before menstruation, with successive application for 10 days. Then, in the fourth menstrual cycle, administration of XFSWF was stopped.
Plasma and urine sample collection
PDM plasma and urine samples were collected on the first day of menstrual cycle for 4 menstrual cycles. Pre-1 samples were collected on the first day of the first menstrual cycle before administration of XFSWF. Post-2 samples were collected on the first day of the second menstrual cycle with XFSWF administration. Post-3 samples were collected on the first day of the third menstrual cycle with XFSWF administration. Post-4 samples were collected on the first day of the fourth menstrual cycle without XFSWF administration.All plasma sample purification was limited to protein precipitation with methanol. One hundred μL plasma samples were extracted with 400 μL methanol (including clarithromycin 2.0 μg mL−1 as internal standard). After vortex for 1min and centrifugation at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min, most protein was removed and the mixture solution was filtered through a PTFE membrane of 0.22 μm.
000 × g for 10 min, most protein was removed and the mixture solution was filtered through a PTFE membrane of 0.22 μm.
Urine samples were thawed at room temperature and centrifuged at 5000 × g for 10 min. The supernatant liquid 1 mL was added to 3 mL methanol (including leucine-enkephalin 2.7 μg mL−1 as internal standard) and vortex mixed for 30 s, then centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min to obtain the supernatant. The supernatant was filtered through 0.22 μm PTFE membrane.
000 × g for 10 min to obtain the supernatant. The supernatant was filtered through 0.22 μm PTFE membrane.
All samples were transferred to an autosampler vial kept at 4 °C and an aliquot of 5 μL was injected for LC/MS analysis.
Plasma laboratory tests
Prostaglandin F2alpha (PGF2α), leukotriene B4 (LTB4), nitric oxide (NO), oxytocin (OT), vasopressin (VAP), 5-hydroxytryptamine (5-HT) and glucocorticoid (GC) were detected by the ELISA assay.37–39 Plasma testosterone (T) was measured by chemiluminescence.40 Biochemical assays were used to analyze plasma lipoprotein (a) [S-LP(a)], activated partial thromboplastin time (APTT), Fibrinogen (Fib) and complete blood count (CBC).UPLC-QTOF/MS and UPLC-QqQ/MS conditions
Samples were run in a random, nongrouped order. The UPLC analysis was performed on a Waters ACQUITY UPLC system (Waters Corporation, Milford, USA). Acquity UPLC BEH-C18 column (2.1 mm × 100 mm, 1.7 μm) was applied for all analyses. The mobile phase was composed of acetonitrile (A) and 0.1% formic acid, v/v (B). For plasma analysis, the mobile phase with a linear gradient elution: 0–0.5 min, A: 5%; 0.5–24 min, A: 5–100%; 24–28 min, A: 100%; 28–30 min, A: 100–5%. For urine analysis, the mobile phase with a linear gradient elution: 0–2.5 min, A: 2%; 2.5–10 min, A: 10–20%; 10–11.5 min, A: 20–40%; 11.5–16 min, A: 40–80%; 16–18 min, A: 80–2%.The flow rate of the mobile phase was 0.4 mL min−1, and the column temperature was maintained at 35 °C.
MS was performed on a Synapt™ Q–TOF (Waters, Manchester, UK). The instrument was operated by using an electrospray ionization (ESI) source in positive mode. The ionization source conditions were as follows: capillary voltage of 3.0 kV, source temperature of 120 °C and desolvation temperature of 350 °C. The sampling cone voltage was set at 30 V, extraction cone was 2.0 V (for plasma sample) or 0.7 V (for urine sample), trap collision energy was 6.0 V, transfer collision energy 4.0 V, trap gas flow was 1.50 mL min−1, ion energy was at 1.0 V. Nitrogen and argon were used as cone and collision gases, respectively. The cone and desolvation gas flow were 50 and 600 L h−1, respectively. The scan time of 0.5 s (for plasma sample) or 0.2 s (for urine sample) was used throughout with interval scan time of 0.02 s and with collision energy of 6 eV. The MS data were collected from m/z 100 to 1000 Da in positive ion in centroid mode. Data acquisition and processing were performed by using Masslynx™ v 4.1 (Waters Corporation).
Leucine–enkephalin was used as the lock mass generating an [M + H]+ ion (m/z 556.2771) and [M − H]− ion (m/z 554.2615) at a concentration of 200 pg mL−1 and flow rate of 100 μL min−1 to ensure accuracy during the MS analysis. Dynamic range enhancement was applied throughout the MS experiment to ensure accurate mass measurement over a wider dynamic range.
UPLC-QqQ/MS was used for quantitative determination of the principal markers. Chromatographic analysis was performed with UPLC-QTOF/MS.
MS detection was performed using a Xevo Triple Quadrupole MS (Waters Corp., Milford, MA) equipped with an ESI source. ESI-MS spectra were acquired in positive ion multiple reaction monitoring (MRM) mode. The conditions of MS analysis were designed as follows: the capillary voltage at 2 kV. Desolvation gas flow rate was set to 1000 L h−1 at a temperature of 550 °C. Cone gas flow rate was set at 50 L h−1 and the source temperature at 150 °C. Cone voltage (CV) and collision energy (CE) were set depending upon each specific MRM for each marker. The dwell time was automatically set by the MassLynx software.
In addition, 10 plasma (or urine) samples were randomly selected from each group and mixed together as the quality control (QC) sample. The QC sample was used to optimize the condition of UPLC-QTOF/MS, as it contained the most information of whole plasma (or urine) samples. Every day, after the instrument was calibrated, the QC sample was first analyzed to test the stability of the instrument, making sure the instrument was in the same condition during the whole analytical procedure.
Multivariate statistical analysis
Multivariate statistical analysis in the form of principal components analysis (PCA) and orthogonal partial least-squared discriminant analysis (OPLS-DA) was carried out. SIMCA-P 11.0 (Umetrics AB, Umeå, Sweden) was used for the chemometric analysis. The quality of the model was described by the cross-validation parameter Q2, indicating the predictability of the model, and R2Y, which represents the total explained variation for the X matrix. S-plot and variable importance in the projection (VIP) were used for the selection of potential biomarkers. Variables with VIP values larger than 1 were chosen to be more important for the explanation of Y (response).41Pearson correlation coefficients were calculated with SPSS v18.0 (IBM SPSS, USA). The correlation heat-map was built and optimized with MATLAB software (MathWorks, US). Based on a sample of paired data (Xi, Yi), the sample Pearson correlation coefficient is  , where
, where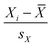 ,
, ![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) and SX are the standard score, sample mean, and sample standard deviation, respectively. The correlation coefficient is always between −1 and +1. The closer the correlation is to ±1, the closer to a perfect linear relationship.
and SX are the standard score, sample mean, and sample standard deviation, respectively. The correlation coefficient is always between −1 and +1. The closer the correlation is to ±1, the closer to a perfect linear relationship.
Univariate statistical analysis
The most important discriminatory metabolite resonances, as determined by PLS–DA loadings plots, were integrated and normalized to the sum of the total spectral integral. The ratios of peak area of each marker to the internal standard (IS) were used for quantitative comparison between control, case and treatment groups.Using SPSS v18.0, differences between the PDM patients pre-treatment, post-treatment and healthy control groups were analyzed using the Mann Whitney and ANOVA test, assuming non-normal data distribution; p-values < 0.05 were considered significant.
Results and discussion
Among the twenty-five patients, four patients were excluded in the first menstrual cycle as they refused to take medicine or due to other disease. XFSWF was ineffective in four of twenty-one patients. The mean VAS score in the 4th menstrual cycle was 6.2 ± 0.5, while in the 1st menstrual cycle, it was 6.5 ± 1.0. The scores means showed no significant differences in pre- and post- treatment (p > 0.05). Seventeen patients obtained effective treatment. The mean VAS score in the 4th menstrual cycle was 3.0 ± 1.7, while in the 1st menstrual cycle, it was 6.2 ± 1.1. The VAS score decreased after treatment and was significantly different compared with that of the pre-treatment (p < 0.001). The clinical effective rate of XFSWF was 81.0%.Plasma biochemical analysis
Prostaglandins, vasopressin, oxytocin and neurotransmitters were usually considered to assess PDM degree and treatment efficacy.42,43 Here, more indexes with NEI system were analyzed in plasma from the pre- and post-treatment PDM subjects. A paired-samples T test was used to show the significant changes. In the seventeen patients with effective treatment, plasma PGF2α, PGE2, LTB4, NO, OT, VAP, 5-HT, GC, T, S-LP(a), APTT, Fib and MCHC levels in the pre-treatment PDM subject at the 1st menstrual cycle (Pre-1) were significantly different compared to the post-treatment PDM subject at the 4th menstrual cycle (Post-4) (Table 2). XFSWF could obviously reduce levels of PGF2α, PGE2, LTB4, OT, VAP, 5-HT, T, S-LP(a), increase levels of NO, GC, APTT, Fib and MCHC in post-treatment PDM subject at the 4th menstrual cycle compared to the 1st menstrual cycle. In the four patients with ineffective treatment, levels of these tested indexes showed no significant difference by self control (p > 0.05).| Classify | Indexes | Pre-1 | Post-4 | p-Values |
|---|---|---|---|---|
| Inflammatory mediators | Prostaglandin F2α (PGF2α, ng mL−1) | 44.47 ± 18.99 | 17.82 ± 10.23 | 0.012 |
| Leukotriene B4 (LTB4, pg mL−1) | 864.97 ± 318.96 | 524.23 ± 171.49 | 0.024 | |
| Nitric oxide (NO, μmol L−1) | 57.97 ± 20.23 | 100.98 ± 26.92 | 0.017 | |
| Neuro transmitters | Oxytocinum (OT, pg mL−1) | 290.82 ± 121.35 | 140.04 ± 27.29 | 0.010 |
| Vasopressin (VAP, pg mL−1) | 599.51 ± 302.32 | 187.66 ± 57.39 | 0.011 | |
| 5-hydroxytryptamine (5-HT, ng mL−1) | 160.25 ± 72.58 | 71.85 ± 29.79 | 0.011 | |
| Endocrine hormone | Testosterone (T, nmol L−1) | 1.17 ± 0.68 | 0.79 ± 0.37 | 0.036 |
| Glucocorticoid (GC, pg mL−1) | 334.57 ± 86.51 | 545.48 ± 133.10 | 0.012 | |
| Blood related mediators | Lipoprotein (a) [S-LP(a), ng mL−1] | 247.62 ± 82.80 | 147.81 ± 41.97 | 0.012 |
| APTT (s) | 29.84 ± 3.53 | 33.49 ± 3.07 | 0.001 | |
| Fibrinogen (Fib, g L−1) | 2.57 ± 0.30 | 2.83 ± 0.44 | 0.045 | |
| MCHC (g L−1) | 330.95 ± 8.79 | 338.14 ± 10.37 | 0.020 |
These indexes could be considered as important biochemistry factors to clinical diagnosis and treatment.
Multivariate data analysis for blood plasma and urine
Plasma and urine from seventeen patients who participated in the trial with effective treatment were used for the metabonomics analysis.Representative plasma and urine spectra from the pre-treatment PDM subject (sample collected at the 1st menstrual cycle) are displayed in Fig. 1.
 | ||
| Fig. 1 Representative base peak intensity (BPI) chromatograms of plasma and urine samples collected in patients before XFSWF derived from UPLC-Q-TOF/MS. (A) Plasma sample. (B) Urine sample. | ||
After multivariate statistical analysis using a PCA model, a total of 616 (plasma samples) and 644 (urine samples) signals in positive modes were detected from the PDM subjects (XFSWF pre-treatment) and healthy controls.
Preliminary PCA scores plots showed clear clustering of pre-1 samples versus healthy controls samples (Fig. 2A and 3A), which demonstrated that the PDM pathological process caused changes in biofluid metabolites. Based on these results, a supervised OPLS-DA model was built to find potential biomarkers for PDM. OPLS-DA distinguished PDM and healthy control cohorts with 100% sensitivity and no less than 95% specificity using a leave one out algorithm. R2Y of this OPLS-DA model was 0.894 (plasma samples) and 0.987 (urine samples) respectively. Q2 was 0.704 (plasma samples) and 0.964 (urine samples) (Fig. 2B and 3B). Using a full external validation paradigm, discriminatory sensitivity was 100% and specificity no less than 90%.
 | ||
Fig. 2 Principal components analysis (PCA) scores plots for plasma samples of (A) pre-treatment PDM subject (red  ) versus healthy controls (green ) versus healthy controls (green  ) and orthogonal partial least-squared discriminant analysis (OPLS-DA) scores plots of (B) pre-treatment PDM subject (red ) and orthogonal partial least-squared discriminant analysis (OPLS-DA) scores plots of (B) pre-treatment PDM subject (red  ) versus healthy controls (green ) versus healthy controls (green  ). (C) S-plot of OPLS-DA and (D) VIP-plot of OPLS-DA of PDM in positive mode. ). (C) S-plot of OPLS-DA and (D) VIP-plot of OPLS-DA of PDM in positive mode. | ||
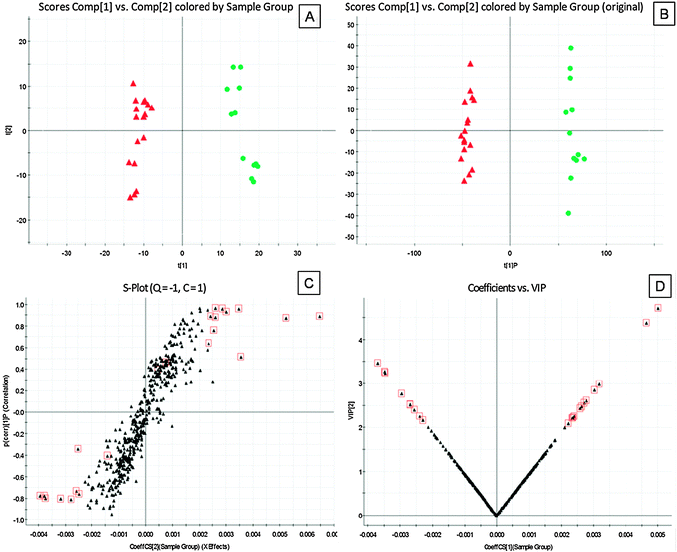 | ||
Fig. 3 Principal components analysis (PCA) scores plots for urine samples of (A) pre-treatment PDM subject (red  ) versus healthy controls (green ) versus healthy controls (green  ) and orthogonal partial least-squared discriminant analysis (OPLS-DA) scores plots of (B) pre-treatment PDM subject (red ) and orthogonal partial least-squared discriminant analysis (OPLS-DA) scores plots of (B) pre-treatment PDM subject (red  ) versus healthy controls (green ) versus healthy controls (green  ). (C) S-plot of OPLS-DA and (D) VIP-plot of OPLS-DA of PDM in positive mode. ). (C) S-plot of OPLS-DA and (D) VIP-plot of OPLS-DA of PDM in positive mode. | ||
From the S-plots, various metabolites could be identified as being responsible for the separation between pre-1 sample and healthy controls. Variables would then be selected as candidates when their VIP values were larger than 2. A variable would be rejected without the support of its confidence interval. Combining the results of the S-plot and VIP-value plot (Fig. 2C, D, 3C and D), the UPLC-QTOF/MS analysis platform provided the retention time, precise molecular mass and MS/MS data for the structural identification of biomarkers.
The precise molecular mass was determined within measurement errors (<5 ppm) by QTOF; meanwhile, the potential elemental composition, degree of unsaturation and fractional isotope abundance of compounds were obtained. The presumed molecular formula was searched in Human Metabolome Database (http://hmdb.ca/) and other databases to identify the possible chemical constitutions. MS/MS data were screened to determine the potential structures of the ions. Finally, reference compounds were used to confirm some of the identified markers.
As an example, we took m/z 496.3 to illustrate the marker identification process. At first, the accurate mass of the potential marker was determined: its corresponding peak was made out according to its retention time in total ion chromatogram from ESI+ scan, then an accurate mass of the marker ([M + H]+ at m/z 496.3498) was found from the mass spectrum. Secondly, particular MS/MS information about fragmentation pattern of the marker was acquired from the Q–TOF system. Under positive ion mode, MS/MS figure contains fragment ion [M + H–H2O]+ (m/z 478.3), [M + H–C5H13NO4P]+ (m/z 313.2), [M + H–C19H37NO2]+ (m/z 184.0). It can be inferred that this marker might be a lysophosphatidylcholine (LPC). Finally, the database of Human Metabolome was searched based on the clues we got from the above process. As a result, the marker was identified as LysoPC(16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) (Fig. S2, ESI†). The literature data were also used to confirm the result.44
0) (Fig. S2, ESI†). The literature data were also used to confirm the result.44
According to the protocol detailed above, 15 endogenous metabolites, such as phytosphingosine, sphingosine, dihydrocortisol, estrone, 17-hydroxyprogesterone, myristoylglycine, palmitoylglycine and LPCs, contributing to the separation of the pre-1 sample and health controls were detected in the samples (Table 3).
| No | tR (min) | m/z | Mass accuracy a (ppm) | MS/MS | Metabolites | Biological pathwayc | Content varianced | Source |
|---|---|---|---|---|---|---|---|---|
| a Mass accuracy (ppm) was calculated according to the exact mass on the Pubchem website.b Confirmed by standard samples.c A, sphingolipid metabolism; B, glycerophospholipid metabolism; C, steroid hormone biosynthesis.d Compared with healthy controls. (↑) up-regulated. (↓): down-regulated. | ||||||||
| 1 | 11.71 | 318.3025 | 0.53 | 256.2 [M + H–C2H5O2]+ | Phytosphingosine | A | ↓ | Plasma |
| 184.0 [M + H–C6H16NO2]+ | ||||||||
| 2 | 13.67 | 518.3289 | 0.83 | 184.0 [M + H–C16H32NO4P]+ | LysoPC (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) | B | ↑ | Plasma |
| 125.0 [M + H–C24H42NO3]+ | ||||||||
| 3 | 14.16 | 494.3297 | 1.0 | 311.2 [M + H–C5H13NO4P]+ | LysoPC (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) | B | ↑ | Plasma |
| 184.0 [M + H–C19H35NO2]+ | ||||||||
| 4 | 14.44 | 544.3356 | −0.86 | 217.1 [M + H–C14H34NO5P]+ | LysoPC (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) | B | ↑ | Plasma |
| 184.0 [M + H–C19H38NO3]+ | ||||||||
| 5 | 14.75 | 520.3314 | −1.7 | 502.3 [M + H–H2O]+ | LysoPC (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) | B | ↑ | Plasma |
| 184.0 [M + H–C21H37NO2]+ | ||||||||
| 6 | 15.64 | 496.3446 | 8.7 | 313.2 [M + H–C5H13NO4P]+ | LysoPC (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0)b 0)b | B | ↑ | Plasma |
| 184.0 [M + H–C19H37NO2]+ | ||||||||
| 7 | 16.05 | 522.3575 | 2.9 | 445.2 [M + H–C3H8NO]+ | LysoPC (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) | B | ↑ | Plasma |
| 184.0 [M + H–C21H39NO2]+ | ||||||||
| 8 | 9.25 | 271.1681 | −6.3 | 147.1 [M + H–C8H12O]+ | Estroneb | C | ↑ | Urine |
| 9 | 11.14 | 331.2192 | −0.91 | 195.1 [M + H–C9H12O]+ | 17-Hydroxyprogesterone | C | ↑ | Urine |
| 138.0 [M + H–C12H17O2]+ | ||||||||
| 10 | 11.27 | 286.2366 | −5.6 | 227.1 [M + H–C2H2O2]+ | Myristoylglycine | B | ↑ | Urine |
| 11 | 11.78 | 365.2338 | 2.7 | 223.1 [M + H–C9H18O]+ | Dihydrocortisolb | C | ↓ | Urine |
| 12 | 12.78 | 314.2708 | 4.1 | 255.2 [M + H–C2H2O2]+ | Palmitoylglycine | B | ↑ | Urine |
| 13 | 13.75 | 318.3006 | −0.63 | 256.2 [M + H–C2H5O2]+ | Phytosphingosine | A | ↓ | Urine |
| 146.1 [M + H–C11H23O]+ | ||||||||
| 14 | 15.15 | 338.2432 | 7.1 | 281.2 [M + K–C2H3NO]+ | Sphingosine | A | ↓ | Urine |
| 15 | 16.74 | 413.2567 | 6.0 | 184.0 [M + H–C9H12NO4P]+ | LysoPC (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) | B | ↑ | Urine |
As shown in Table 3, these identified metabolites participated in three pathways, including sphingolipid metabolism, glycerophospholipid metabolism and steroid hormone biosynthesis.
Metabolic changes correlated with XFSWF intervention
To determine whether XFSWF was possible to influence the metabolic pattern of the PDM patients, we constructed a PCA model based on UPLC-QTOF/MS data of plasma and urine samples. As shown in PCA scores plot (Fig. 4), separation of the pre-1 sample and control group was clearly achieved, which indicated that the endogenous biomarkers altered significantly in the pathological process of PDM. After treatment of two menstrual cycles, PDM subjects relocated closely to healthy controls group, which indicated a significant intervention of XFSWF for endogenous biomarkers of PDM. | ||
Fig. 4 PCA scores plots of (A) PDM plasma samples and (B) PDM urine samples. Pre-1: the first menstrual cycle before administration of XFSWF (red  ), Post-2: XFSWF administration for one menstrual cycle (purple ), Post-2: XFSWF administration for one menstrual cycle (purple  ), Post-3: XFSWF administration for two menstrual cycles (dark red ), Post-3: XFSWF administration for two menstrual cycles (dark red  ), Post-4: XFSWF administration for three menstrual cycles (dark green ), Post-4: XFSWF administration for three menstrual cycles (dark green  ); healthy controls (green ); healthy controls (green  ) ) | ||
PCA and PLS–DA plot represents a visualized result for the filtration of specific potential biomarkers, but QTOF/MS had disadvantage in the quantitative analysis. Contents change of some metabolites could not be accurately presented. Therefore, QqQ/MS was used for quantification of the above 15 metabolites described identified in pre-1, post-4 and healthy controls group. MRM transitions and parameters applied in the study are listed in Table S3 (ESI†). ANOVA and paired-sample t test were used to compare the relative concentrations (the ratio of peak area to IS) of 15 metabolites in Pre-1, Post-4 and healthy controls group (Table 4).
| No | Metabolites | Pre-1 | Post-4 | Healthy controls |
|---|---|---|---|---|
| Pre-1: the first menstrual cycle before administration of XFSWF. Post-4: XFSWF administration for three menstrual cycles. *p < 0.05, **p < 0.01, ***p < 0.001 vs. healthy controls. #p < 0.05, ##p < 0.01, ###p < 0.001 post-4 vs. pre-1 group. | ||||
| 1 | Phytosphingosine | 2.856 ± 0.368* | 3.729 ± 0.736# | 3.957 ± 1.253 |
| 2 | LysoPC(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) | 1.309 ± 0.352 | 1.206 ± 0.496 | 1.265 ± 0.338 |
| 3 | LysoPC(16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) | 4.142 ± 0.821** | 3.223 ± 1.162# | 3.294 ± 0.849 |
| 4 | LysoPC(20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) | 88.89 ± 10.43** | 72.87 ± 11.01## | 72.09 ± 10.60 |
| 5 | LysoPC(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) | 76.45 ± 9.21* | 68.58 ± 10.30## | 67.25 ± 8.545 |
| 6 | LysoPC(16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) | 94.30 ± 15.80* | 77.94 ± 18.91## | 80.26 ± 6.92 |
| 7 | LysoPC(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) | 146.5 ± 21.4* | 127.3 ± 25.94## | 130.8 ± 5.5 |
| 8 | Estrone | 233.6 ± 108.0* | 181.1 ± 81.3# | 201.3 ± 43.4 |
| 9 | 17-Hydroxyprogesterone | 14.87 ± 6.38** | 10.80 ± 1.59# | 9.986 ± 0.835 |
| 10 | Myristoylglycine | 78.38 ± 11.19*** | 19.08 ± 5.57### | 16.27 ± 5.84 |
| 11 | Dihydrocortisol | 20.50 ± 7.80** | 55.96 ± 4.56## | 58.87 ± 4.04 |
| 12 | Palmitoylglycine | 28.49 ± 5.92* | 23.00 ± 3.23# | 20.75 ± 7.88 |
| 13 | Phytosphingosine | 17.67 ± 4.13* | 30.47 ± 11.81## | 25.04 ± 5.51 |
| 14 | Sphingosine | 8.264 ± 2.872** | 15.73 ± 3.67# | 12.87 ± 3.68 |
| 15 | LysoPC(10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) | 53.13 ± 10.65** | 25.09 ± 3.05## | 24.78 ± 4.08 |
LysoPC(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was not considered as biomarker, for its concentration showed no significant differences between pre-1 group and healthy controls based on QqQ/MS data. Concentration of LPCs increased significantly in patients compared with healthy controls. Estrone, 17-Hydroxyprogesterone, myristoylglycine and palmitoylglycine showed the same trend with LPCs. Phytosphingosine, found in both plasma and urine, decreased significantly in patients compared with healthy controls. Dihydrocortisol and sphingosine in urine also significantly decreased in patients compared with healthy controls.
3) was not considered as biomarker, for its concentration showed no significant differences between pre-1 group and healthy controls based on QqQ/MS data. Concentration of LPCs increased significantly in patients compared with healthy controls. Estrone, 17-Hydroxyprogesterone, myristoylglycine and palmitoylglycine showed the same trend with LPCs. Phytosphingosine, found in both plasma and urine, decreased significantly in patients compared with healthy controls. Dihydrocortisol and sphingosine in urine also significantly decreased in patients compared with healthy controls.
After administration of XFSWF for three menstrual cycles, the 15 potential biomarkers recovered to that in healthy controls (p > 0.05). As a result, LPCs and steroid hormones could be considered as potential biomarkers of PDM and targets for XFSWF.
Correlation network of markers
In the -omic era and NEI system, it is important to monitor changes in the abundance of a whole suite of different biomarkers and biochemistry factors.Elucidation of the relationship between metabolites (biomarkers) and physiological function (biochemistry factors) would be informative for clinical diagnosis, medical treatment or pathophysiology research. Correlation networks was a suitable method in this step.34 Based on the alteration of all potential biomarkers and important biochemistry factors, correlation networks were built to find out the inner correlations among these important substances. The substances were connected according to their Pearson correlation coefficient (r) and the significance of the connection was set at the p < 0.05 level.
A correlation heat-map showed the systemic substance changes of PDM and XFSWF intervention. As shown in Fig. 5, PGF2α, OT and T positively correlated with LysoPC (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), LysoPC (10
0), LysoPC (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and myristoylglycine (r > 0.6), negatively correlated with dihydrocortisol (r < −0.6). VAP, 5-HT and S-LP(a) positively correlated with myristoylglycine (r > 0.6), negatively correlated with dihydrocortisol (r < −0.6). NO, APTT and MCHC positively correlated with dihydrocortisol (r > 0.6), negatively correlated with myristoylglycine (r < −0.6). Fib positively correlated with phytosphingosine (r = 0.82). GC positively correlated with dihydrocortisol (r = 0.77). Correlation of biomarkers and biochemistry factors reflected that lycerophospholipid, sphingolipid and steroid hormone biosynthesis metabolism participate in the pathological process of PDM, affect XFSWF efficacy.
0) and myristoylglycine (r > 0.6), negatively correlated with dihydrocortisol (r < −0.6). VAP, 5-HT and S-LP(a) positively correlated with myristoylglycine (r > 0.6), negatively correlated with dihydrocortisol (r < −0.6). NO, APTT and MCHC positively correlated with dihydrocortisol (r > 0.6), negatively correlated with myristoylglycine (r < −0.6). Fib positively correlated with phytosphingosine (r = 0.82). GC positively correlated with dihydrocortisol (r = 0.77). Correlation of biomarkers and biochemistry factors reflected that lycerophospholipid, sphingolipid and steroid hormone biosynthesis metabolism participate in the pathological process of PDM, affect XFSWF efficacy.
 | ||
| Fig. 5 Correlation analysis between biomarkers and biochemistry factors. Color key indicates correlated value, blue: negatively correlated, red: positively correlated. | ||
This result may indicate that the profile of the metabolomics was closely correlated to traditional biological and clinical end points, and was thus becoming a useful tool for diagnosis and evaluation of therapy.
PDM is a heterogeneous disorder with uncertain etiology, different clinical and biochemical phenotypes in affected individuals. One historically documented formula Four-Agents-Decoction (Si Wu Tang) had effectiveness to PDM associated with no adverse syndrome.45 XFSWF is a formula derived from Four-Agents-Decoction. Our study showed that the clinical effective rate of XFSWF was 81.0%. In this study, an MS-based metabonomics approach was applied for analyzing blood plasma and urine biomarkers in PDM patients, as well as the possible metabolic mechanism of XFSWF. Several biomarkers involved in the pathology of PDM, such as sphingolipid (phytosphingosine, sphingosine), dihydrocortisol, estrone, 17-hydroxyprogesterone, myristoylglycine, palmitoylglycine and LPCs.
The potential biomarkers discovered can be classified into three categories. Category 1 was sphingolipids (phytosphingosine, sphingosine), which are ubiquitous components of cellular membranes in eukaryotic cells and in a few bacteria.46 Phytosphingosine are converted into ceramide and its phosphate derivative, sphingosine 1 phosphate (S1P). Ceramide converted into sphingosine and sphingomyelins.47 The reduced amount of phytosphingosine and sphingosine might either reflect a reduction of the synthesis of these metabolites, or implicate their rapid consumption because of the increased synthesis of sphingomyelins or S1P. S1P plays an important role during development, particularly in vascular maturation and has been implicated in pathophysiology of cancer, wound healing, and atherosclerosis.48 Plasma sphingolipids level has been found to be a risk factor for polycystic ovary syndrome and endometriosis.49,50
Category 2 was glycerophospholipids, which are precursors for lipid mediators involved in signal transduction processes. Degradation of glycerophospholipids by phospholipase A(2) (PLA(2)) generates arachidonic acid (AA) and lysophosphatidylcholine (LPC). Arachidonic acid is then stereospecifically oxygenated through cyclooxygenase (COX) to prostaglandins (PGs) or through lipoxygenase (LOX) enzymes to leukotrienes.51 LPC is in turn enzymatically converted to lysophosphatidic acid (LPA).52 It has been found to have some functions in cell signaling and specific receptors (coupled to G proteins).53 LPA signaling can also have pathological consequences, influencing aspects of endometriosis and ovarian cancer.54 The present findings imply that decreased sphingolipids and increased glycerophospholipids may lead to the increased risk of secondary dysmenorrhea (SDM) in PDM patients.
Category 3 was steroid hormone. Hormone is an important marker in the causation of PDM. The clinical end points were consistent with the level change of metabolites.
After XFSWF treatment for four menstrual cycles, the endogenous metabolites profile (biomarkers) showed no significantly different compared to healthy controls. Clinical end points showed statistically significant difference before and after treatment. Furthermore, sphingolipids such as phytosphingosine correlated more positive tightly with Fib. Glycerophospholipids such as myristoylglycine, lysoPC (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) correlated more tightly with S-LP(a), 5-HT, VAP, PGF2α, OT, T, MCHC, NO and APTT. Dihydrocortisol correlated more tightly with GC. These indicated that impairment of homeostasis of the neural, endocrinal and immune system was an important aspect of PDM pathophysiology and XFSWF adjusted the system integrity. Glycerophospholipid, sphingolipid and steroid hormone biosynthesis metabolism participated in physiological functions and affected the drug efficacy. A pathway analysis of the identified biomarkers of PDM is shown in Fig. 6.
0) correlated more tightly with S-LP(a), 5-HT, VAP, PGF2α, OT, T, MCHC, NO and APTT. Dihydrocortisol correlated more tightly with GC. These indicated that impairment of homeostasis of the neural, endocrinal and immune system was an important aspect of PDM pathophysiology and XFSWF adjusted the system integrity. Glycerophospholipid, sphingolipid and steroid hormone biosynthesis metabolism participated in physiological functions and affected the drug efficacy. A pathway analysis of the identified biomarkers of PDM is shown in Fig. 6.
 | ||
| Fig. 6 A pathway analysis of the identified biomarkers and biochemistry factors for PDM and XFSWF intervention. | ||
Conclusions
The present study investigated metabolic variations in patients with PDM using MS-based metabonomics and a traditional Chinese herbal formula intervention. Clear metabolic differences were observed between PDM patients and healthy controls. These variations involved significant perturbations in glycerophospholipid metabolism, sphingolipid metabolism and steroid hormone biosynthesis. The metabolic deviations decreased with XFSWF intervention. XFSWF can be used for the treatment of PDM cases, especially for those adolescents who do not desire a contraceptive method or to reduce the risk of SDM.The present study also demonstrated that metabonomics is a powerful tool in the study of PDM, providing information on changes in metabolites and NEI pathways that will aid investigation of the complex pathogenesis and development of new methods for clinical diagnosis and treatment.
Acknowledgements
This research was financially supported by National Natural Science Foundation of China (81202880), Natural Science Foundation of Jiangsu Province, China (BK2012456) and Key Research Project in Basic Science of Jiangsu College and University of China (No. 06KJA36022). It was also a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (ysxk-2010). We are also pleased to thank Waters China Ltd. for technical support.References
- S. V. Doubova, H. R. Morales, S. F. Hernandez, M. del Carmen Martinez-Garcia, M. G. de Cossio Ortiz, M. A. Soto, E. R. Arce and X. Lozoya, J. Ethnopharmacol., 2007, 110, 305–310 CrossRef.
- S. E. Daniels, S. Torri and P. J. Desjardins, J. Gen. Intern. Med., 2005, 20, 62–67 CrossRef.
- Y. M. Sun, L. Wang and G. Li, Tianjin J. Tradit. Chin. Med., 2009, 26, 367–369 Search PubMed.
- L. Q. Zheng, J. L. Bi, C. W. Zhan, W. Deng, J. S. Lin, R. Luo and X. S. Zhao, Guiding J. Tradit. Chin. Med. Pharm., 2011, 17, 27–29 Search PubMed.
- G. C. Whittle, P. Slade and C. M. Ronalds, J. Psychosom. Res., 1987, 31, 79–84 CrossRef CAS.
- J. Teperi and M. Rimpela, Soc. Sci. Med., 1989, 29, 163–169 CrossRef CAS.
- A. S. Coco, Am. Fam. Physician., 1999, 60, 489–496 CAS.
- Z. Harel, Expert. Opin. Pharmacother., 2008, 9, 2661–2672 CrossRef CAS.
- E. M. Jun, S. Chang, D. H. Kang and S. Kim, Int. J. Nurs. Stud., 2007, 44, 973–981 CrossRef.
- W. Y. Zhang and A. Li Wan Po, Br. J. Obstet. Gynaecol., 1998, 105, 780–789 CrossRef CAS.
- C. S. Hsu, J. K. Yang and L. L. Yang, Phytomedicine, 2006, 13, 94–100 CrossRef.
- J. C. Ai, Shaanxi J. Tradit. Chin. Med., 1993, 14, 5–6 Search PubMed.
- P. Liu, L. Ye, J. A. Duan, E. X. Shang, S. L. Su, X. S. Fan and Y. P. Tang, China J. Tradit. Chine. Med. Pharm., 2011, 26, 138–140 Search PubMed.
- Z. B. Wang and Y. J. Sheng, J. Tradit. Chin. Med., 1997, 38, 423 Search PubMed.
- P. Liu, J. A. Duan, Y. Q. Hua, Y. P. Tang, X. Yao and S. L. Su, J. Ethnopharmacol., 2011, 133, 591–597 CrossRef.
- Y. Q. Hua, J. A. Duan, S. L. Su, Y. Lu, Q. J. Wang and Y. P. Tang, J. China Pharm. Univ., 2008, 39, 72–76 Search PubMed.
- P. Liu, J. A. Duan, R. Liu, J. M. Guo and Y. P. Tang, China J. Traditi. Chin. Med. Pharm., 2011, 26, 902–907 CAS.
- S. L. Su, J. A. Duan, X. H. Zhao, P. F. Hou, E. X. Shang, Y. P. Tang, A. W. Ding and Y. Q. Hua, World Science and Technology/Modernization of Traditional Chinese Medicine and Materia Medica, 2008, 10, 50–57 Search PubMed.
- R. Liedman, S. R. Hansson, D. Howe, S. Igidbashian, A. McLeod, R. J. Russell and M. Akerlund, Gynecol. Endocrinol., 2008, 24, 508–513 CrossRef CAS.
- S. Nigam, C. Benedetto, M. Zonca, I. Leo-Rossberg, H. Lubbert and J. Hammerstein, Eicosanoids, 1991, 4, 137–141 CAS.
- S. Li, Z. Q. Zhang, L. J. Wu, X. G. Zhang, Y. D. Li and Y. Y. Wang, IET Syst. Biol., 2007, 1, 51–60 CrossRef CAS.
- T. Ma, C. G. Tan, H. Zhang, M. Q. Wang, W. J. Ding and S. Li, Mol. Biosyst., 2010, 6, 613–619 RSC.
- J. K. Nicholson, J. R. Everett and J. C. Lindon, Expert Opin. Drug Metab. Toxicol., 2012, 8, 135–139 CrossRef CAS.
- T. A. Clayton, J. C. Lindon, O. Cloarec, H. Antti, C. Charuel, G. Hanton, J. P. Provost, J. L. Le Net, D. Baker, R. J. Walley, J. R. Everett and J. K. Nicholson, Nature, 2006, 440, 1073–1077 CrossRef CAS.
- E. Stener-Victorin, G. Holm, F. Labrie, L. Nilsson, P. O. Janson and C. Ohlsson, J. Clin. Endocrinol. Metab., 2010, 95, 810–819 CrossRef CAS.
- A. K. Arakaki, J. Skolnick and J. F. McDonald, Nature, 2008, 456, 443–443 CrossRef CAS.
- E. Holmes, R. L. Loo, J. Stamler, M. Bictash, I. K. S. Yap, Q. Chan, T. Ebbels, M. De Iorio, I. J. Brown, K. A. Veselkov, M. L. Daviglus, H. Kesteloot, H. Ueshima, L. C. Zhao, J. K. Nicholson and P. Elliott, Nature, 2008, 453, 396–U350 CrossRef CAS.
- Y. P. Qiu, G. X. Cai, M. M. Su, T. L. Chen, X. J. Zheng, Y. Xu, Y. Ni, A. H. Zhao, L. X. Xu, S. J. Cai and W. Jia, J. Proteome Res., 2009, 8, 4844–4850 CrossRef CAS.
- M. I. F. Shariff, A. I. Gomaa, I. J. Cox, M. Patel, H. R. T. Williams, M. M. E. Crossey, A. V. Thillainayagam, H. C. Thomas, I. Waked, S. A. Khan and S. D. Taylor-Robinson, J. Proteome Res., 2011, 10, 1828–1836 CrossRef CAS.
- E. J. Want, A. Nordstrom, H. Morita and G. Siuzdak, J. Proteome Res., 2007, 6, 459–468 CrossRef CAS.
- H. C. Keun, Pharmacol. Ther., 2006, 109, 92–106 CrossRef CAS.
- J. Nielsen and S. Oliver, Trends Biotechnol., 2005, 23, 544–546 CrossRef CAS.
- E. M. Lenz and I. D. Wilson, J. Proteome Res., 2007, 6, 443–458 CrossRef CAS.
- C. H. Tu, D. M. Niddam, H. T. Chao, L. F. Chen, Y. S. Chen, Y. T. Wu, T. C. Yeh, J. F. Lirng and J. C. Hsieh, Pain, 2010, 150, 462–468 CrossRef.
- M. E. Wewers and N. K. Lowe, Res. Nurs. Health, 1990, 13, 227–236 CrossRef CAS.
- M. Sarkari, J. Brown, X. Chen, S. Swinnea, R. O. Williams 3rd and K. P. Johnston, Int. J. Pharm., 2002, 243, 17–31 CrossRef CAS.
- P. L. Durham, C. V. Vause, F. Derosier, S. McDonald, R. Cady and V. Martin, Headache, 2010, 50, 844–851 CrossRef.
- D. K. Miller, J. D. Menke, N. S. Hayes, A. Uzieblo, D. Tew, Y. Hayashi, Y. Guan, A. Zhao, R. T. Cummings, Y. W. Park and T. T. Yamin, Anal. Biochem., 2006, 349, 129–135 CrossRef CAS.
- H. Genc, H. Uzun, A. Benian, G. Simsek, R. Gelisgen, R. Madazli and O. Guralp, Arch. Gynecol. Obstet., 2011, 284, 1367–1373 CrossRef CAS.
- L. E. Hooper, K. E. Foster-Schubert, D. S. Weigle, B. Sorensen, C. M. Ulrich and A. McTiernan, Nutr. Res., 2010, 30, 163–170 CrossRef CAS.
- P. Y. Yin, D. F. Wan, C. X. Zhao, J. Chen, X. J. Zhao, W. Z. Wang, X. Lu, S. L. Yang, J. R. Gu and G. W. Xu, Mol. Biosyst., 2009, 5, 868–876 RSC.
- G. Creatsas, E. Deligeoroglou, A. Zachari, D. Loutradis, T. Papadimitriou, K. Miras and D. Aravantinos, Eur. J. Obstet. Gynecol. Reprod. Biol., 1990, 36, 292–298 CrossRef CAS.
- R. Liedman, S. R. Hansson, D. Howe, S. Igidbashian, R. J. Russell and M. Akerlund, Eur. J. Obstet. Gynecol. Reprod. Biol., 2008, 137, 189–192 CrossRef CAS.
- X. J. Wang, H. Y. Wang, A. H. Zhang, X. Lu, H. Sun, H. Dong and P. Wang, J. Proteome Res., 2012, 11, 1284–1301 CrossRef CAS.
- L. L. L. Yeh, J. Y. Liu, K. S. Lin, Y. S. Liu, J. M. Chiou, K. Y. Liang, T. F. Tsai, L. H. Wang, C. T. Chen and C. Y. Huang, PLoS One, 2007, 2, e719 Search PubMed.
- D. V. Lynch and T. M. Dunn, New Phytol., 2004, 161, 677–702 CrossRef CAS.
- C. R. Gault, L. M. Obeid and Y. A. Hannun, Adv. Exp. Med. Biol., 2010, 688, 1–23 CrossRef CAS.
- K. Watterson, H. Sankala, S. Milstien and S. Spiegel, Prog. Lipid Res., 2003, 42, 344–357 CrossRef CAS.
- P. Santulli, L. Marcellin, J. C. Noel, B. Borghese, I. Fayt, D. Vaiman, C. Chapron and C. Mehats, Fertil. Steril., 2012, 97, 904–911 CrossRef CAS.
- L. Sun, W. Hu, Q. Liu, Q. Hao, B. Sun, Q. Zhang, S. Mao, J. Qiao and X. Yan, J. Proteome Res., 2012, 11, 2937–2946 CrossRef CAS.
- H. N. Jabbour, K. J. Sales, R. D. Catalano and J. E. Norman, Reproduction, 2009, 138, 903–919 CrossRef CAS.
- M. P. Wymann and R. Schneiter, Nat. Rev. Mol. Cell Bio., 2008, 9, 162–176 CrossRef CAS.
- J. H. Kabarowski, Prostaglandins Other Lipid Mediators, 2009, 89, 73–81 CrossRef CAS.
- X. Q. Ye and J. Chun, Trends Endocrinol. Metab., 2010, 21, 17–24 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2mb25238d |
| This journal is © The Royal Society of Chemistry 2013 |
