Research highlights
Yu-Shik Hwanga, Hojae Baeb, Mohsen Akbaricde, Mehmet R. Dokmecicd and Ali Khademhosseini*acdef
aDepartment of Maxillofacial Biomedical Engineering, Institute of Oral Biology, School of Dentistry, Kyung Hee University, 1 Hoegidong, Dongdaemun-gu, Seoul 130-701, Republic of Korea
bCollege of Animal Bioscience and Technology, Department of Bioindustrial Technologies, Konkuk University, Hwayang-dong, Kwangjin-gu, Seoul 143-701, Republic of Korea
cCenter for Biomedical Engineering, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Cambridge, Massachusetts 02139, USA. E-mail: alik@rics.bwh.harvard.edu
dHarvard–MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA
eWyss Institute for Biologically Inspired Engineering, Harvard University, Boston, Massachusetts 02115, USA
fWorld Premier International – Advanced Institute for Materials Research (WPI-AIMR), Tohoku University, Sendai 980-8577, Japan
First published on 8th October 2013
Microwells with a degradable cover for single cell studies
Due to the inherent heterogeneity in biological cells there is a need to analyze single cell responses to their surrounding environment. Cellular heterogeneity is important in many biological responses such as studies related to stem cells, cancer and immunology.1 Unfortunately, most conventional biological assays fail to provide information about individual cells and are limited to population responses. Hence, there is a need to develop technologies that enable single cell tracking and analysis in a manner that is simple and scalable for large cell numbers. Microfabricated wells have emerged as one possible way to isolate individual cells.2 However, most approaches using microwell platforms provide open well systems which are prone to cross-contamination due to the material exchange between microwells during the flow of culture medium.To overcome the limitations associated with standard microwell assays, Liu and colleagues3 have recently introduced a method for immobilizing single cell assays within microwells with a programmable cover by combining a microwell culture platform with a permeable enzyme-triggered DNA hydrogel. In their work, Jin et al.3 first fabricated a poly(dimethylsiloxane) (PDMS) microwell array as a basic cell culture platform, where the individual cells were hydrodynamically trapped inside each microwell. These single cells were next enclosed inside the microwells by covering the PDMS microwell array with a DNA hydrogel-based film. They could also be released from the microwells in a controlled manner via restriction enzyme-mediated DNA hydrogel degradation (Fig. 1A).
 | ||
| Fig. 1 Schematic illustration of a permeable enzyme-triggered DNA hydrogel and the microwell culture platform for single cell assays. (A) The closing and opening process for trapping single cells inside microwells. (B) Basic mechanism of hybridization of the Y-shaped DNA unit and the cleavage of the DNA scaffold. Figure inspired from Jin et al.3 | ||
The DNA hydrogel was composed of a Y-shaped DNA unit and a linker, which was made simply by assembling multiple DNA strands. The sticky ends of the Y-shaped DNA unit and the small DNA fragment (linker) contained complementary sequences. The hybridization between these complementary sequences was achieved by vortexing to generate a 200 μm thick DNA hydrogel (Fig. 1B).3 The fabricated DNA hydrogel cover enabled individual cells to be safely confined inside the microwells during the washing steps. A fluorescein-based diffusion test showed adequate permeability of the hydrogel cover to keep the enclosed cells viable during culture.
To enable cell release, the microwells could be opened through restriction enzyme-mediated degradation of the hydrogel cover (Fig. 1B).3 The particular restriction enzyme used here, EcoRI, is known to recognize specific DNA sequences, so called “restriction sites”, and to cleave the DNA containing restriction sites.4 Jin et al.3 integrated a restriction site for EcoRI into the DNA linker, such that the incubation of the hydrogel with EcoRI at 37 °C for 20 minutes resulted in gel degradation and release of the cells from the microwells.
This approach presents an enabling technology for creating single cell assays to capture and monitor individual cells in a controlled manner. The authors' strategy provides a versatile approach to study and understand microenvironment-induced changes in cellular behavior. Furthermore, the introduction of the DNA hydrogel as a smart biomaterial enables potential applications in cell patterning and high-throughput screening for bioengineering as well as in cell encapsulation for tissue engineering.
Ultra fast isolation of circulating tumor cells inside spiral microfluidics
Circulating tumor cells (CTCs) are cells that navigate in the blood stream which are shed from primary or secondary tumors and are known to participate in the metastatic spread of carcinoma.5 Rapid, accurate, and precise detection and isolation of CTCs is essential in understanding their biological relevance in cancer prognosis and treatment. However, the extremely rare population of CTCs (only a few among several million white blood cells (WBCs) and billions of red blood cells (RBCs) in 1 mL of patient blood) demands a new high-efficiency isolation and detection strategy for improved CTC enrichment yield and high sensitivity detection.6 Most current approaches for CTC enrichment are based on affinity binding of CTCs to antibody-conjugated (magnetic) particles.7 Specifically, this strategy relies on cell surface antigens, such as the epithelial cell adhesion molecule (EpCAM) known to be expressed in CTCs, however, the expression of epithelial markers was found to be down-regulated or lost in some aggressive CTC cells undergoing epithelial–mesenchymal transition (EMT).8 Therefore, affinity binding-based methods for CTC enrichment are limited to a narrow range of cancers, prompting the need for novel microfluidic technologies for CTC isolation and enrichment. Numerous technical issues, however, stand in the way of these new approaches, e.g. clogging of channels, low yields in cell recovery, poor cell viability due to the need for relatively long processing times and the need for external forces to overcome high fluidic resistance on-chip.9Han, Lim and colleagues10 have recently developed a spiral microfluidic device for high-throughput isolation and enrichment of CTCs. The spiral microfluidic device created by Warkiani et al.10 consisted of an 8-loop spiral microchannel with a trapezoidal cross-section of 80/130 μm in inner/outer heights. The trapezoidal channel was designed to improve the precision of the cell size-based separation by modifying the flow velocity field. Namely, inside the rectangular channels, the smaller cells migrated to the outer walls, while larger cells remained flowing along the lower inner walls (Fig. 2).10 At the end of the chip, the spiral microchannel was divided into two channels, which enabled the separation of smaller cells from the larger cells where each type was collected at a different outlet port (Fig. 2).
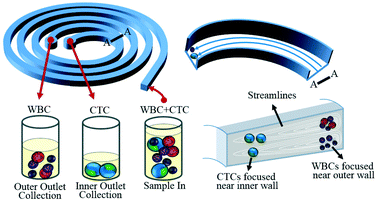 | ||
| Fig. 2 The spiral microfluidic device with a trapezoidal shaped channel for high-throughput cell size-based separation and retrieval of CTCs from blood. Figure adapted and reprinted with permission from the Royal Society of Chemistry from Warkiani et al.10 | ||
To evaluate the performance of the developed spiral microfluidic device in terms of its efficiency in cell separation and recovery, the group tested three cancer cell lines (MCF-7, T24, MDA-MB-231) by suspending roughly 500 cells in 7.5 mL of blood. After RBC lysis, the mixture was passed through the spiral microfluidic device at 1700 μL min−1 with a total processing time of 8 minutes, which resulted in average recovery rates of above 80% for all cell lines, and a high cell viability. Furthermore, to demonstrate the clinical significance of this microfluidic device, blood samples from 5 healthy donors, 5 patients with metastatic breast cancer (MBC) and 5 patients with non-small cell lung cancer (NSCLC) were also tested. The CTCs were successfully isolated from all patient samples with a range of 6–57 CTCs mL−1 for the MBC sample and 3–125 CTCs mL−1 for the NSCLC samples, which was confirmed by their positive reaction with FITC–pan cytokeratin antibody (cancer/epithelial biomarker). The negative samples yielded no false positives.
The spiral microfluidic device had a high yield and facile recovery of CTCs, and provided a reproducible way to collect CTCs for cancer diagnostics. Further technical progress and optimization may help in integrating this microfluidic CTC detection technology into clinical diagnostic and treatment platforms. In addition, the trapezoidal cross-section microfluidic channel shows great promise in applications requiring cell size-based separations in various fields.
Microfluidics for energy: steam-on-a-chip for oil recovery
Currently, the petroleum industry can recover roughly 35% of oils from natural reservoirs. Around 300 million additional barrels could potentially be liberated by increasing the oil recovery factor to above 40%, according to the International Energy Agency (IEA). Hence, enhanced oil recovery (EOR) is essential for improving the total oil production. Existing approaches for EOR include steam assisted, water assisted and hydraulic fracturing methods.11 Steam assisted gravity drainage (SAGD) is the main technology for enhanced recovery of crude oil and bitumen from deep reservoirs, which have high permeability. In this process, two aligned horizontal wells with an approximate length of 1 km are drilled into an oil reservoir, one almost 5 m above the other. Steam is injected from the top well into the reservoir, where it heats up the fluids by releasing its latent heat, resulting in reduced viscosity of oil and other fluids such as bitumen. The heated fluids drain into the lower well due to gravity, where they are pumped out. The main challenge in using SAGD is to operate this technology at optimized conditions that yield maximum economic returns. Therefore, there is an emerging need for developing miniature and well-controlled models that can better mimic the process and provide useful information about the heat transfer and two-phase flow within the reservoirs.Lab-on-chip technologies have emerged from simple flow manipulation devices that perform chemical reactions and have found many applications in other areas including energy. Several microfluidic devices have been developed for biofuel production and micro fuel cells.12,13 Microfluidic models have also been developed to study carbon sequestration in saline aquifers and traditional oil recovery processes.14,15 In a recent study, de Haas et al.16 developed a microfluidic platform to investigate the dynamics of an oil reservoir at the microscale. Their device consisted of a micropillar array fabricated on a glass substrate, a steam generator, temperature and flow controllers, and an imaging system including a USB microscope and a webcam (Fig. 3). The glass microchip was fabricated by wet etching and was designed to have pillars with the same dimensions as the sand grains in the reservoirs with a porosity of 67.7% and 72.5%. To produce the required steam, water was heated while passing through the steel tube and the generated steam was then fed into the chip. The steam temperature was regulated at 100 °C with a temperature controller at the chip inlet. A webcam was put in place such that the entire chip could be monitored during the process. To visualize the dynamics of the flow in the chip at the microscale, a USB microscope was utilized.
 | ||
| Fig. 3 A schematic of the experimental setup for modelling steam assisted gravity drainage in oil reservoirs. The inset image shows the water-in-bitumen droplet formation during the process. Figure adapted and reprinted with permission from the Royal Society of Chemistry from de Haas et al.16 | ||
Bitumen drainage from the microchip was monitored in real time, and the effect of alkaline additives to the steam on the efficiency of the SAGD process was quantified. The results indicate that using alkaline additive steam enhances the recovery effectiveness by ~50% compared to pure steam. In addition, the drainage rate in the alkaline case was 35%–67% faster than the rate with pure steam. Adding alkaline to the steam made the glass surface more hydrophobic, resulting in smaller oil-in-water emulsions. The results of the study indicated that the size of oil-in-water emulsions was reduced from 150 μm in the pure steam case to 6 μm in the additive case. This significant reduction also contributed to the enhancement in the recovery efficiency.
This study shows that lab-on-chip technologies are moving into new areas by expanding their scope to more exciting applications such as oil recovery and sustainable energy use. In the present study microfluidics was used as a powerful tool for modeling the SAGD process in oil recovery. The proposed platform may be further improved to better mimic the structure of actual oil reservoirs. For example, fine clay particles could be added to the bitumen to model certain ores. Furthermore, the entire process could be optimized to determine the most efficient operating conditions. This could be achieved by performing a comprehensive parametric study on the effect of salient parameters such as the pore size, the reservoir porosity and the steam temperature on the overall effectiveness of the SAGD process.
References
- T. Graf and M. Stadtfeld, Cell Stem Cell, 2008, 3, 480–483 CrossRef CAS PubMed.
- S. Lindstrom and H. Andersson-Svahn, Methods Mol. Biol., 2012, 853, 41–52 Search PubMed.
- J. Jin, Y. Xing, Y. Xi, X. Liu, T. Zhou, X. Ma, Z. Yang, S. Wang and D. Liu, Adv. Mater., 2013, 25, 4714–4717 CrossRef CAS PubMed.
- R. J. Roberts, Crit. Rev. Biochem. Mol. Biol., 1976, 4, 123–164 CrossRef CAS.
- S. C. P. Williams, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 4861 CrossRef CAS PubMed.
- S. K. Arya, B. Lim and A. R. A. Rahman, Lab Chip, 2013, 13, 1995–2027 RSC.
- A. Ring, I. E. Smith and M. Dowsett, Lancet Oncol., 2004, 5, 79–88 CrossRef.
- S. Mocellin, U. Keilholz, C. R. Rossi and D. Nitti, Trends Mol. Med., 2006, 12, 130–139 CrossRef CAS PubMed.
- J. Chen, J. Li and Y. Sun, Lab Chip, 2012, 12, 1753–1767 RSC.
- M. E. Warkiani, G. Guan, K. B. Luan, W. C. Lee, A. A. S. Bhagat, P. Kant Chaudhuri, D. S.-W. Tan, W. T. Lim, S. C. Lee, P. C. Y. Chen, C. T. Lim and J. Han, Lab Chip, 2014 10.1039/c3lc50617g.
- M. Bavière, Basic concepts in enhanced oil recovery processes, Elsevier Applied Science, Essex, England, UK, 1991 Search PubMed.
- D. Erickson, D. Sinton and D. Psaltis, Nat. Photonics, 2011, 5, 583–590 CrossRef CAS.
- E. Kjeang, N. Djilali and D. Sinton, J. Power Sources, 2009, 186, 353–369 CrossRef CAS PubMed.
- A. Sell, H. Fadaei, M. Kim and D. Sinton, Environ. Sci. Technol., 2012, 47, 71–78 CrossRef PubMed.
- N. S. K. Gunda, B. Bera, N. K. Karadimitriou, S. K. Mitra and S. M. Hassanizadeh, Lab Chip, 2011, 11, 3785–3792 RSC.
- T. W. de Haas, H. Fadaei, U. Guerrero and D. Sinton, Lab Chip, 2013, 13, 3832–3899 RSC.
| This journal is © The Royal Society of Chemistry 2013 |
