Microfluidic opportunities in the field of nutrition
Sixing Liab, Justin Kiehnea, Lawrence I. Sinowayc, Craig E. Cameronbd and Tony Jun Huang*ab
aDepartment of Engineering Science and Mechanics, The Pennsylvania State University, University Park, PA 16802, USA. E-mail: junhuang@psu.edu; Fax: 814-865-9974; Tel: 814-863-4209
bCell and Developmental Biology (CDB) Graduate Program, The Huck Institutes of the Life Sciences, The Pennsylvania State University, University Park, PA 16802, USA
cHeart and Vascular Institute and Department of Medicine, Penn State College of Medicine, and Milton S. Hershey Medical Center, Hershey, PA 17033, USA
dDepartment of Biochemistry and Molecular Biology, The Pennsylvania State University, University Park, PA 16802, USA
First published on 10th September 2013
Abstract
Nutrition has always been closely related to human health, which is a constant motivational force driving research in a variety of disciplines. Over the years, the rapidly emerging field of microfluidics has been pushing forward the healthcare industry with the development of microfluidic-based, point-of-care (POC) diagnostic devices. Though a great deal of work has been done in developing microfluidic platforms for disease diagnoses, potential microfluidic applications in the field of nutrition remain largely unexplored. In this Focus article, we would like to investigate the potential chances for microfluidics in the field of nutrition. We will first highlight some of the recent advances in microfluidic blood analysis systems that have the capacity to detect biomarkers of nutrition. Then we will examine existing examples of microfluidic devices for the detection of specific biomarkers of nutrition or nutrient content in food. Finally, we will discuss the challenges in this field and provide some insight into the future of applied microfluidics in nutrition.
Introduction
Proper dietary nutrition is imperative to healthy child growth and maintenance of human wellbeing. Nutritional disorders, whether resulting from excessive (overnutrition) or inadequate (undernutrition) nutrient intake, are classified as malnutrition. Worldwide, malnutrition is directly or indirectly associated with various major causes of death.1,2 In developed countries, overnutrition is a major health risk because it can lead to obesity and diseases such as diabetes and cardiovascular disease. Between 2009 and 2010, more than one-third of all adults in the United States were classified as obese.3 As standards of living increase in developing countries this trend repeats itself, rendering obesity a global health challenge.4While the world is faced with this increasing prevalence of overnutrition and obesity, undernutrition remains a major public health concern, affecting more than 900 million people worldwide.5 Maternal and child undernutrition, highly prevalent in underdeveloped countries, is particularly malignant because it can substantially increase the mortality and disease burden of young children.6 As shown in Fig. 1, the influence of child undernutrition is most drastic in south-central Asia and eastern Africa where stunting, underweight, and wasting resulting from severe macronutrient malnutrition contribute to the majority of child deaths.7 Globally, stunting, severe wasting, and intrauterine growth restriction cause more than 2.2 million deaths each year and lead to 21% of disability-adjusted life-years (DALYs) for children under 5 years of age.6 In addition to macronutrient malnutrition, micronutrient deficiencies are widespread, affecting about one third of the world's population and causing a variety of adverse effects on human health.8
 | ||
| Fig. 1 Latest country prevalence estimates for stunting, underweight and wasting among children under-five years of age. Images reproduced from ref. 7 with permissions from WHO and UNICEF. | ||
Global action has already been taken to address this worldwide malnutrition challenge. International commitments to eliminate stunting, underweight and wasting of children, such as the United Nation’s (UN) Zero Hunger Challenge, have been launched. In addition, food-based strategies, such as dietary diversification, food fortification, and micronutrient supplementation, have been widely adopted to tackle micronutrient deficiencies.8 While global or regional nutrition reinforcement can be achieved through adequate food supply and micronutrient supplementation, effective approaches for accurately evaluating nutritional status and dietary nutrient content are still lacking.
For nutritional screening and malnutrition diagnosis, anthropometric indicators (body measurements), biochemical indicators (biomarkers) and clinical signs are often recommended.9 Currently, the Malnutrition Universal Screening Tool (MUST) is still the major method of diagnosing malnutrition.10 Relying on anthropometric indicators such as height, weight, body mass index (BMI), and unplanned weight loss, it lacks specificity and does not adequately include biomarkers for reliable assessment of micronutrient deficiency in early stages. In order to diagnose micronutrient deficiencies before the effects are severe enough to cause perceptible symptoms, blood or urine tests are often utilized to measure specific micronutrient biomarkers. Unfortunately, conventional blood or urine tests must be carried out in centralized or regional laboratories capable of maintaining bulky, expensive equipment. The necessity of sample transport, along with long sample analysis times, makes real-time diagnoses in POC settings very difficult. This difficulty is compounded in poor and rural areas where the risk of malnutrition is highest and access to medical equipment and personnel is lowest. Additionally, high costs make it unlikely that conventional blood or urine tests can be scaled up for nutritional screening at the population level. Therefore, the development of portable, inexpensive devices that can efficiently detect biomarkers of nutrition will significantly benefit the global effort in fighting against malnutrition.
In addition to the detection of nutritional biomarkers, microfluidic platforms can be used to monitor the nutrient content in food and micronutrient supplements. Conventional nutrient analysis techniques, utilized by the food industry, are ill-suited for use when food supplies and micronutrient supplements are being distributed in resource-limited regions, as is the case in the UN's Zero Hunger Challenge. This also calls for the development of small-size, low-cost, easy-to-use devices that can sensitively analyze multiple types of nutrients.
Given these constraints, microfluidic technologies seem to have great potential for applicability. With its capacity to precisely handle small volumes of liquid, microfluidics has transformed the way biomedical and chemical assays are designed and executed.11 Despite the extensive and fruitful work that has been done over the past two decades, the field of nutrition is still largely unexplored by the microfluidics community. Thus, the purpose of this Focus article is to invoke interest among microfluidic researchers in the field of nutrition. In the following sections we will highlight some of the recent advances in microfluidic blood analysis systems that have the capacity to detect biomarkers of nutrition. Then we will examine existing examples of microfluidic devices for the detection of specific biomarkers of nutrition or nutrient content in food. Finally, we will discuss the challenges in this field and provide some insight into the future of applied microfluidics in nutrition.
Microfluidic blood analysis platforms
With the advantages of small size, low cost, ease of integration and automation, microfluidics technology is expected to revolutionize the healthcare industry with portable, inexpensive, and high-performance medical devices for POC diagnostics.12–16 Because human blood contains massive amounts of diagnostic information, miniaturization of blood analysis is an area of intense interest for the microfluidics community. Many researchers have devoted themselves to developing microfluidic platforms for the purpose of tackling specific human health problems through blood analysis. The integrated blood barcode chip (IBBC) developed by the Heath group is one such example.17 Through the integration of a densely patterned antibody microarray based on the DNA-encoded antibody library (DEAL) techniques,18 this IBBC device is capable of multiplexed detection of serum proteins in minutes from only a finger prick of blood. Furthermore, the Sia group recently miniaturized the standard enzyme-linked immunosorbent assay (ELISA) in a microfluidic platform to develop a portable device specifically for HIV and syphilis diagnosis in developing countries.19 In a field-testing in Rwanda, this device was used to analyze hundreds of locally collected human samples, demonstrating performance comparable to its benchtop counterparts.Despite recent innovations and significant potential, microfluidics has yet to live up to lofty expectations. Oftentimes microfluidic devices are designed and tested in laboratory settings where external connections (tubing, optical fiber, etc.) and components (power supply, compressed air, pumping systems, etc.) are taken for granted. This can be an obstacle when the devices are to be applied in remote settings. Learning from the widely used home pregnancy test kits, we believe that connection-free, self-powered, and highly integrated microfluidic blood analysis devices will be better-suited for POC diagnostics.
Several groups have taken steps towards this goal by developing self-powered, self-contained microfluidic blood analysis devices.20–23 As shown in Fig. 2(a), the Lee group developed a stand-alone, self-powered integrated microfluidic blood analysis system (SIMBAS).23 This connection-free, vacuum-powered device can achieve fluid propulsion, plasma separation, and biomarker detection on a single chip. To demonstrate the capabilities of the device, the authors analyzed whole blood samples spiked with various concentrations of biotin (vitamin B7). Their results show that 5 μL of whole blood can be analyzed in 10 min, with a limit of detection of approximately 1.5 pM.
 | ||
| Fig. 2 (a) Schematics of the stand-alone, self-powered integrated microfluidic blood analysis system (SIMBAS); (b) Disc design of the fully automated LOD ELISA system; (c) The brief workflow of the μPAD used for multiplexed transaminase test. Images reproduced from ref. 23, 34 and 55 with permissions from the Royal Society of Chemistry and the American Association for the Advancement of Science. | ||
In addition to merely modifying conventional PDMS microfluidic chips, researchers have endeavored to introduce innovative microfluidic formats to achieve connection-free, automated, and integrated microfluidic devices. Centrifugal microfluidics, or a lab-on-a-disc (LOD) system, is one particularly promising, alternative microfluidic format. Although the concept was first brought to the public as early as 1969,24 it was not until Abaxis introduced its Piccolo system in the early 1990s that the diagnostic potential of LOD systems became well recognized. While efforts to commercialize centrifugal microfluidic platforms have persisted, research has pushed forward, continuing to expand the functions and applications of LOD blood analysis systems since the 2000s.25–36
Though the significant advances in LOD technologies have been reviewed extensively in other works,37,38 we will briefly highlight one example of a recently developed platform to illustrate that a connection-free, integrated blood analysis system can be accomplished using the LOD technology. Fig. 2(b) presents the disc design of a fully automated LOD ELISA system developed by the Cho group for the diagnosis of infectious diseases from whole blood.34 Like other LOD platforms, the centrifugal forces generated during disc spinning drive sample flow. This centrifugal pumping requires only an integrated motor to generate spin, minimizing the amount of necessary external components. In addition, preloading of reagents into the microfabricated channel network eliminates the need for external tubing or sample pretreatment. In this work, the authors utilized novel laser irradiated ferrowax microvalves (LIFMs)35,36 in the LOD system for fluid control. By exposing the iron oxide nanoparticle-incorporated paraffin wax to low-intensity laser light, one can precisely melt the wax to control fluid transfer. The LIFM makes it possible to execute the high-speed spinning necessary for plasma separation while maintaining the ability to execute further operations through the confinement of the sample during each individual step. This is also the basis for fully automating the immunoassay with the preset spin program. To demonstrate the functionality of this device, the authors conducted microbead-based suspension ELISA assays to detect the antigen and antibody of Hepatitis B Virus (HBV). The total assay time was reduced from the 2 h necessary for conventional ELISA to less than 30 min while maintaining a comparable limit of detection. In their later work, the Cho group achieved simultaneous biochemical assay and immunoassay33 or multiplexed immunoassays28 in a single integrated LOD device, thereby further demonstrating the great application potential of LOD devices in POC blood analysis.
Paper microfluidics, or microfluidic paper analytical devices (μPAD), provides another innovative format for connection-free microfluidic platforms. Pioneered by the Whitesides group, paper microfluidics has made significant strides despite the technology's very recent introduction. The potential of paper microfluidics in developing low-cost, POC diagnostic tools has been well recognized by the microfluidics community, demonstrated by the rapid research advancement seen in recent years.39–55
In-depth analysis of paper microfluidics is available in other review papers56–58 and is not our focus in this review. Instead, we will elucidate the potential of paper microfluidics in realizing low-cost, connection-free, easy-to-use blood analytical platforms with the use of one recent example. Shown in Fig. 2(c), the μPAD newly developed by the Whitesides group is capable of multiplexed detection of two major liver function indicators, aspartate aminotransferase (AST) and alanine aminotransferase (ALT).55 Requiring only a finger prick of whole blood, the device is capable of performing simultaneous assays in 15 min. Capillary forces are utilized to drive the fluid flow, rendering the platform pump- and connection-free. By patterning hydrophobic barriers onto the layered paper device, the authors are able to create three-dimensional hydrophilic microchannels to realize more complex fluid manipulations such as splitting, mixing, and filtration. Moreover, this three-dimensional fluid network also enables the μPAD to run multiplexed assays with unique conditions and no risk of cross-reactivity between assays. The clinical test results show that the accuracy of the μPAD is comparable to the current gold standard automated methods. In addition to simplicity and accuracy, the device is extremely low-cost, light-weight, and disposable, making it perfect for POC settings.
Microfluidic detection of nutritional biomarkers
Although biomarker detections have not been as popular in the field of nutrition as in disease diagnoses, the importance of proper biomarkers in nutritional screening and malnutrition diagnosis has evoked attention recently.59,60 Direct measurement of biomarkers of nutrition in bodily samples such as blood and urine provides more specific and less biased diagnostic information than anthropometric indicators (body measurements). Moreover, seemingly healthy individuals can unknowingly lack vital nutrients in their diets, eventually leading to disease. In these cases, the detection of biomarkers of nutrition can help diagnose malnutrition in early stages. Portable devices that can specifically and sensitively detect biomarkers of nutrition will greatly enhance nutritional monitoring targeting real-time and home-based evaluation. In this section, we will discuss the work that has already been done in microfluidics regarding the detection of nutritional biomarkers.Macronutrients
Carbohydrate, protein, and fat are classified as macronutrients. The excess consumption of macronutrients is a major cause of obesity, while chronic, insufficient intake of macronutrients leads to severe protein-energy malnutrition. The most successful example of POC detection of macronutrient levels is blood glucose detection. Low-cost, pocket-sized glucose meters are easily accessible in stores. However, because blood glucose levels are more closely tied to diabetes than to nutritional status, we will not discuss microfluidic glucose detection due to the limited length of this Focus article. Instead, we will focus on the detection of biomarkers related to protein and fat status.Historically, the measurement of serum hepatic proteins (albumin, prealbumin, transferrin, etc.) has been used to assess protein nutritional status. Although controversy has arisen recently about the accuracy of using serum hepatic proteins in evaluating nutritional status, the detections of serum hepatic proteins together with indicators of inflammation such as C-reactive protein (CRP) can still provide useful information about nutritional status.61–64
Miniaturization of immunoassay is a common strategy for microfluidic protein detection. Laiwattanapaisal et al. has developed an on-chip immunoassay for detecting urinary albumin with performance comparable to conventional spectrophotometric methods.65 However, the immunoassay-based protein detection often means a tedious procedure and high reagent costs. In order to overcome these limitations, the Lee group developed non-immunological urinary albumin sensors, either based on electrochemical detection66 or specific dye binding.67 By modifying the device design and introducing magnetic bead-based chemiluminescence detection, they also detected serum CRP in microfludic systems.68,69 Besides the Lee group, several other groups have also demonstrated the detection of serum CRP in microfluidic devices.28,70–74Fig. 3(a) shows the capillary-driven immunodiagnostic device developed by Gervais and Delamarche for detecting CRP from human serum.74 With the integration of all the functional elements and reagents on-chip, this device achieves one-step immunoassays, demonstrating the potential advantage of microfluidics in simplifying the detection procedure. Their results show that CRP with a concentration of 1 ng mL−1 can be detected from only 5 μL of serum within 14 min.
 | ||
| Fig. 3 (a) The schematic of the one-step, capillary-driven, POC CRP detection device; (b) The microfluidic chip based on nickel oxide nanorods (NRs-NiO) used for the detection of total cholesterol. Images reproduced from ref. 74 and 84 with permissions from the Royal Society of Chemistry. | ||
In addition to albumin and CRP, microfluidic platforms have been developed to detect cortisol,75–77 a steroid hormone reported to be an indicator of protein-energy malnutrition.78 The utilization of portable microfluidic devices can help realize real-time cortisol monitoring to establish diurnal cortisol concentration behavior.75 Moreover, the integration of nanotechnology into microfluidic platforms significantly increases the sensitivity of cortisol detection, enabling ultrasensitive cortisol sensors.76,77
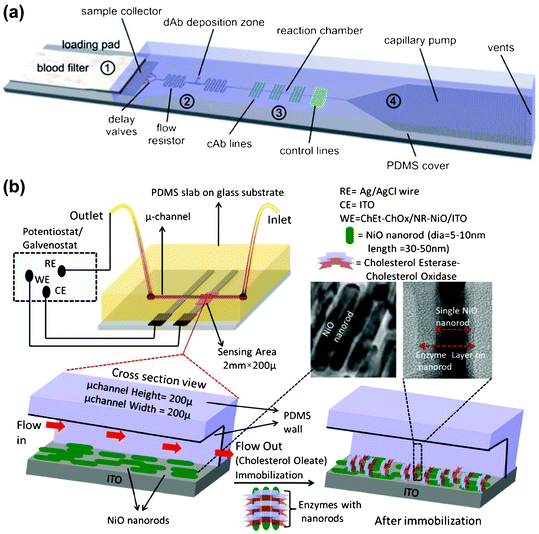
Cholesterol is a lipid with crucial roles in maintaining the integrity of cell membrane, as well as in biosynthesis of steroid hormones and vitamin D. The blood cholesterol level, as an important indicator of fat nutritional status, is also closely related to diseases: increased cholesterol level is believed to increase the risk of cardiovascular diseases while low cholesterol level may increase the risk of cancer, depression, or respiratory diseases. Microfluidic detections of blood cholesterol levels have recently been realized in centrifugal microfluidics33 or paper microfluidics40. Additionally, there is also a recent trend of integrating nanostructured materials into microfluidic devices to realize cholesterol detection.79–84Fig. 3(b) illustrates one recent example of such microfluidic devices for detecting total serum cholesterol.84 In this work, Ali et al. fabricated a microfluidic nano chip utilizing nickel oxide nanorods (NRs-NiO) immobilized with cholesterol esterase (ChEt) and cholesterol oxidase (ChOx). The electrons generated during the enzymatic reactions were transferred to NRs-NiO, resulting in an electrochemical signal indicating total serum cholesterol concentration. Their results show that the use of NRs-NiO gives the device a wide detection range and high sensitivity.
Micronutrients
Compared with macronutrient malnutrition, micronutrient deficiencies are not easily diagnosable only based on anthropometric indicators, unless they are severe enough to cause obvious illness symptoms. Thus the detection of biomarkers of micronutrient status should significantly enhance the diagnosis of micronutrient deficiency. In industry, the potential of microfluidic technology in realizing real-time, POC monitoring of micronutrient status has already been recognized. NanoSpeed Diagnostics Inc. has introduced a series of Test4™ products, which are basically Lateral Flow Immunoassay (LIFA)-based portable devices. Among the products, the Test4D™, Test4Ca™ and Test4Fe™ are capable of measuring vitamin D, calcium, and iron levels in human blood, respectively. Meanwhile, recent progress has also been made in academia in developing microfluidic systems for measuring micronutrient levels in blood.Iron deficiency is one of the most common micronutrient deficiencies around the world and causes iron-deficiency anemia. Besides serum iron level, hemoglobin and serum ferritin are widely used indicators of iron status. Steigert et al. developed a LOD platform based on colorimetric assay that can directly measure the concentration of hemoglobin from only 2 μL of whole blood within 100 s.85 Microfluidic detections of serum ferritin have also been reported by several groups using on-chip immunoassays.86–88 Through the integration of pneumatic microvalves, Kartalov et al. realized a high-throughput, multi-antigen microfluidic fluorescence immunoassay system, which can simultaneously measure serum ferritin, CRP and several other serum proteins.88
Recently, the first monolithically fabricated microfluidic electrochemical sensor capable of measuring zinc in serum was reported.89 In this work, microfabricated bismuth electrodes interact with the zinc in the sample to generate quantitative information about zinc levels in blood serum treated with HCl. This reported microfluidic sensor requires only 200 μL of blood sample and shows good sensitivity in the 5 mM to 50 mM range in pH 6 sodium acetate buffer.
In addition to detection of trace elements, microfluidics has been successful in the monitoring of vitamin A status.90 Vitamin A levels are conventionally monitored through characterization of retinol levels in the blood. Due to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio between retinol binding protein (RBP) and retinol, RBP is a suitable surrogate for vitamin A monitoring. Based on this, the on-chip measurement of RBP instead of retinol was employed in this work, speeding up the process and eliminating the need for extensive lab work. To test the functionality of this microfluidic RBP enzyme immunoassay (EIA), the authors executed a field evaluation in a population at risk of vitamin A deficiency using both the microfluidic RBP EIA and the HPLC retinol measurement. The results showed that the microfluidic platform is as reliable as the conventional HPLC method in estimating vitamin A deficiency, while with obvious advantages in terms of portability, low cost, and rapid detection.
1 ratio between retinol binding protein (RBP) and retinol, RBP is a suitable surrogate for vitamin A monitoring. Based on this, the on-chip measurement of RBP instead of retinol was employed in this work, speeding up the process and eliminating the need for extensive lab work. To test the functionality of this microfluidic RBP enzyme immunoassay (EIA), the authors executed a field evaluation in a population at risk of vitamin A deficiency using both the microfluidic RBP EIA and the HPLC retinol measurement. The results showed that the microfluidic platform is as reliable as the conventional HPLC method in estimating vitamin A deficiency, while with obvious advantages in terms of portability, low cost, and rapid detection.
Microfluidic monitoring of nutrient content
In the fight against global malnutrition, food-based strategies are the most straightforward approaches. Providing adequate food supply, micronutrient supplementation, dietary diversification, and food fortification are the simplest and most direct ways to combat malnutrition.8 Therefore, the monitoring of nutrient content in dietary and micronutrient supplementation is a necessity for ensuring the efficiency of these strategies.Conventional methods used in food analysis include mass spectroscopy (MS),91 gas chromatography (GC),92 high-performance liquid chromatography (HPLC),93 capillary electrophoresis (CE),94 and enzyme-linked immunosorbent assay (ELISA).95 Although these analytical methods are widely used in the food industry for quality control and safety inspection, they do not meet the requirements of field use necessary for application in resource-limited areas because they necessitate sophisticated equipment, are relatively time-consuming, and require high reagent costs when conducted at large scales. Therefore, for large-scale, routine monitoring of nutrient content in dietary and micronutrient supplementation where malnutrition is most severe, portable, fast, and low-cost analytical platforms are desirable. Portability, miniaturization, and the capability to manipulate small amounts of fluid make microfluidic technology an ideal match for this type of application. Recently, the potential for microfluidics in food analysis has started to be realized.96–98 In this section, we will review existing applications of microfluidics in monitoring nutrient content.
Microchip capillary electrophoresis
The introduction of microfluidics technology enabled the miniaturization of capillary electrophoresis (CE) and the realization of microchip CE.99 Compared with conventional counterparts, microchip CE has the potential to simultaneously assay multiple small samples very rapidly, thereby significantly increasing throughput, reducing reagent consumption, and decreasing assay time.100 With these advantages, microchip CE is emerging as a useful tool in analysis of carbohydrates, proteins, amino acids, and water-soluble vitamins in food.101,102 The first application of microchip CE in nutrient analysis was protein separation and quantification in fish muscle.103 In this work, protein extract from both farmed and wild-caught fish was analyzed with microchip CE to evaluate the differences in relative protein concentration to assess the impact of aquaculture on fish quality.103 With the integration of light emitting diode-induced fluorescence (LIF) detection, Ueno et al. were able to quantify amino acids in functional foods such as sport beverages, jelly-form beverages, and tablet-form functional foods.104 In another work, Crevillén et al. demonstrated fast analysis of water-soluble vitamins (vitamin B and C) in tablets within 350 s, clearly showcasing the fast-analysis capacity of microchip CE.105 Continuous advances in research in microchip CE have resulted in commercialization by the analytical industry. The Agilent 2100 Bioanalyzer is the first commercially available microchip CE device for DNA, RNA and protein analysis. To compare the performance of this lab-on-a-chip device with conventional CE, Blazek and Caldwell analyzed the protein content of twenty different soybean cultivars with both conventional CE and the Agilent 2100 Bioanalyzer.106 In addition to increased time-efficiency, the lab-on-a-chip device demonstrated better performance in terms of the repeatability of migration times and peak areas.Nanomaterial-enabled microfluidic sensors
Since microfluidic devices usually handle very small sample volumes, ultra-sensitive sensing schemes are necessary to detect limited amounts of analytes. The integration of nanomaterial-based sensors107–109 is a promising solution. Due to their high sensitivity and specificity, nanomaterial-based sensors have already attracted growing attention in food analysis.110 However, the introduction of nanotechnology into microfluidics to analyze low-concentration nutrients in foods is still in its infancy.111–113 One successful example is the use of carbon nanotubes (CNT) in microchip CE to detect water-soluble vitamins in foods.111 In this work, CNT-based electrochemical detection (ED) demonstrated decreased overpotential, enhanced sensitivity, and improved resolution due to the large surface-to-volume ratio, enhanced electronic transfer, and strong sorption capacity of CNT.Surface plasmon resonance (SPR)114–118 biosensors are also versatile tools in various fields. Commercialized SPR biosensors have been available since 1990, when the first SPR instrument was developed by Biacore AB. Recently, SPR biosensors have been applied successfully in food analysis.119 Using a Biacore Q® instrument, Gao et al. successfully quantified several water-soluble vitamins (B2, B12, folic acid, biotin, and pantothenic acid) in infant formula samples.113 In another work, Fernández et al. integrated the gold diffraction grating surface into a six-channel microfludic device to achieve on-site, label-free, multiplexed antibiotics analysis in milk samples.112
Microfluidic bioassays and chemical assays
With its extraordinary capacity for handling small volumes of liquid, microfluidics provides an attractive solution for miniaturizing conventional bioassays and chemical assays.120 By immobilizing L-glutamate dehydrogenase and D-phenylglycine aminotransferase onto the surface of the microchannel or microparticles, Laiwattanapaisal et al. developed two miniature platforms capable of detecting L-glutamate in food samples with a limit of detection of 3 μM.121 Recently, microfluidic chemiluminescence systems for measurements of vitamin B1 and B12 levels in tablets or eggs have also been reported, with very high sensitivity compared to reported methods.122–124 Besides system miniaturization, another distinct merit of microfluidics technology is the ease of parallelization, which can be realized simply by introducing multiple channels. This can increase throughput or enable multiplexed analysis. Hoegger et al. developed a disposable microfluidic ELISA device for the quantification of folic acid in infant formula samples within 5 min, shown in Fig. 4 (a) and (b).125 The detection of folic acid is based on an indirect competitive immunoassay, in which free folic acids in the samples compete with pre-coated folic acids on the channel surface for the binding sites of the antibody-enzyme conjugates. Thus the concentration of folic acids in the food samples is inversely proportional to the amount of antibody-enzyme conjugate bound to the surface-immobilized folic acids, which is detected electrochemically. The integration of eight parallel microchannels allows calibration and analysis in one step. | ||
| Fig. 4 (a) and (b) show the schematic of the microfluidic ELISA device for the quantification of folic acid in infant formula samples. (c) to (h) show the fully integrated, portable microfluidic sedimentation cytometer (SeCy) used for milk analysis. Images reproduced from ref. 125 and 127 with permission from Springer. | ||
In addition to conventional microfluidic devices, newly developed paper-based microfluidics and centrifugal microfluidics technology have been applied in nutrient analysis.126–128Fig. 4 (c) to (h) show an integrated, low-cost, portable microfluidic sedimentation cytometer (SeCy) based on centrifugal microfluidics.127 This device consists of 12 independent flattened funnel-like units fabricated on a single plastic compact disc (CD) for loading 150 μl milk samples. When the CD is rotating, somatic cells and fat globules can be separated for analysis under centrifugal forces, with detection ranges of 2.0–6.5% fat content and 5 × 104 to 5 × 105 cells ml−1 cell counts. This sample-to-answer device can be used for on-site, rapid quality control of milk product.
Conclusions and perspectives
In the previous sections, we highlighted some of the recently developed microfluidic blood analysis platforms and examined the current status of applied microfluidics in the detection of nutritional biomarkers or monitoring of nutrient content. Despite great achievements in disease diagnostics, the application of microfluidics in the field of nutrition is still in its infancy. One reason for this is the limited popularity of biomarkers in nutritional screening and malnutrition diagnosis. It is understandable that before blood or urine tests became affordable and easily accessible, people relied on anthropometric indicators to evaluate the likelihood of existing malnutrition or the risk of developing future malnutrition. However, these anthropometric indicators are subject to influences other than nutritional status and are ill-suited for diagnosing early micronutrient deficiencies. Therefore, biomarkers of nutrition will be useful for overcoming the limitations of anthropometric indicators and facilitating early diagnosis of micronutrient deficiency. The development of portable and affordable diagnostic devices capable of detecting biomarkers of nutrition will thus be necessary and helpful. This unmet need has been realized by the healthcare industry with efforts devoted to this endeavor. For example, Diagnostics For All, a non-profit enterprise saving lives through the creation of low-cost, easy-to-use, POC diagnostic devices specifically for the developing world, is working with MC10 Inc. to combine the low-cost patterned paper-based diagnostics with the flexible electronics platform in order to develop innovative diagnostic devices capable of quantitatively and accurately monitoring micronutrient status in POC settings and at low cost. These affordable and portable microfluidic devices, once realized, will essentially bring nutritional evaluation off bench and enable on-site, real-time assessment of nutritional status especially in remote areas.It is essential to recognize that there are several challenges to applying microfluidics in the field of nutrition. To design POC microfluidic devices for the detection of biomarkers of nutrition, the first decision is what biomarkers should be targeted. Unlike disease diagnoses in which one or a few biomarkers can provide useful information, the evaluation of nutritional status based on biomarkers is more complicated with a continued debate as to which biomarkers are to be used for specific purposes.59,60 Although accumulated knowledge about biomarkers of nutrition will certainly help us make wiser decisions in the future, currently, it seems reasonable to measure multiple biomarkers when no single biomarker is available. In addition, for diagnosing micronutrient deficiency, it is also beneficial to be able to detect multiple micronutrient levels at one time. To fulfill these demands, multiplexed detection seems a reasonable solution. As we have discussed in previous sections, multiplexed detection has been recognized by the community as a characteristic of microfluidic systems. With proper assay design and parallelization, it is possible to develop POC microfluidic devices capable of detecting multiple biomarkers of nutrition. The benefit of such devices is the ability to provide more comprehensive information regarding the overall nutritional status.
As for microfluidic monitoring of nutrient content, the major challenge is the complexity of the food matrix. Multiple steps of sample preparation are often required for the analysis of food samples, often hindering the development of fully integrated systems. Although enormous efforts have been devoted to developing effective food sample preparation techniques, a really satisfying approach is still yet to come. In the meantime, the emerging trend of integrating nanomaterial-based sensors into microfluidics sheds light on tackling this sample preparation problem. The miniaturization nature of microfluidics means that only a very small amount of food sample is required. The large surface area to volume ratio and excellent electron transfer rate of nanomaterials help realize ultrasensitive and ultrafast sensors for monitoring nutrient content from only minute amounts of food samples. Combined together, this may reduce the burden of pretreatment of large amounts of food samples before analysis and lead to a practical microfluidic nutrient content analyzer.
In conclusion, microfluidic technology holds enormous potential in terms of realizing POC, multi-parameter nutritional evaluation and integrated nutrient content analysis devices. Despite this potential, the field of nutrition is still a relatively unexplored area for the microfluidics community. We hope that this article can draw more attention to this field so that we can together push nutrition towards POC settings with microfluidics technology.
Acknowledgements
This research was supported by National Institutes of Health (NIH) Director's New Innovator Award (1DP2OD007209-01), NIH Clinical and Translational Science Award (UL1 TR000127), Huck Innovative & Transformational Seed (HITS) Fund, US Department of Agriculture (USDA/NRI), and the Penn State Center for Nanoscale Science (MRSEC) under Grant DMR-0820404. S.L. and C.E.C. were funded in part by grant AI45818 from NIAID, NIH. Components of this work were conducted at the Penn State node of the NSF-funded National Nanotechnology Infrastructure Network (NNIN).References
- World Health Organization, Global nutrition policy review: what does it take to scale up nutrition action?, 2013 Search PubMed.
- U. E. Schaible and S. H. E. Kaufmann, PLoS Med., 2007, 4, e115 Search PubMed.
- K. M. Flegal, M. D. Carroll, B. K. Kit and C. L. Ogden, JAMA, J. Am. Med. Assoc., 2012, 307, 491 CrossRef PubMed.
- M. Chopra, S. Galbraith and I. Darnton-Hill, Bulletin of the World Health Organization, 2002, 80, 952 Search PubMed.
- V. J. B. Martins, T. M. M. Toledo Florêncio, L. P. Grillo, M. do Carmo P. Franco, P. A. Martins, A. P. G. Clemente, C. D. L. Santos, M. de Fatima A. Vieira and A. L. Sawaya, Int. J. Environ. Res. Public Health, 2011, 8, 1817 CrossRef PubMed.
- R. E. Black, L. H. Allen, Z. A. Bhutta, L. E. Caulfield, M. de Onis, M. Ezzati, C. Mathers and J. Rivera, Lancet, 2008, 371, 243 CrossRef.
- M. de Onis, D. Brown, M. Blössner and E. Borghi, Levels & Trends in Child Malnutrition, 2012 Search PubMed.
- L. Allen, B. de Benoist and O. Dary, Guidelines on food fortification with micronutrients, 2006 Search PubMed.
- M. Blössner and M. de Onis, Malnutrition: quantifying the health impact at national and local levels, 2005 Search PubMed.
- J. Kondrup, S. P. Allison, M. Elia, B. Vellas and M. Plauth, Clin. Nutr., 2003, 22, 415 CrossRef CAS.
- G. M. Whitesides, Nature, 2006, 442, 368 CrossRef CAS PubMed.
- X. Mao and T. J. Huang, Lab Chip, 2012, 12, 1412 RSC.
- P. Neuži, S. Giselbrecht, K. Länge, T. J. Huang and A. Manz, Nat. Rev. Drug Discovery, 2012, 11, 620 CrossRef PubMed.
- Y. Zhao, Z. S. Stratton, F. Guo, M. I. Lapsley, C. Y. Chan, S.-C. S. Lin and T. J. Huang, Lab Chip, 2013, 13, 17 RSC.
- X. Mao and T. J. Huang, Lab Chip, 2012, 12, 4006 RSC.
- P. Li, Z. S. Stratton, M. Dao, J. Ritz and T. J. Huang, Lab Chip, 2013, 13, 602 RSC.
- R. Fan, O. Vermesh, A. Srivastava, B. K. H. Yen, L. Qin, H. Ahmad, G. A. Kwong, C.-C. Liu, J. Gould, L. Hood and J. R. Heath, Nat. Biotechnol., 2008, 26, 1373 CrossRef CAS PubMed.
- R. C. Bailey, G. A. Kwong, C. G. Radu, O. N. Witte and J. R. Heath, J. Am. Chem. Soc., 2007, 129, 1959 CrossRef CAS PubMed.
- C. D. Chin, T. Laksanasopin, Y. K. Cheung, D. Steinmiller, V. Linder, H. Parsa, J. Wang, H. Moore, R. Rouse, G. Umviligihozo, E. Karita, L. Mwambarangwe, S. L. Braunstein, J. van de Wijgert, R. Sahabo, J. E. Justman, W. El-Sadr and S. K. Sia, Nat. Med., 2011, 17, 1015 CrossRef CAS PubMed.
- K. Hosokawa, M. Omata, K. Sato and M. Maeda, Lab Chip, 2006, 6, 236 RSC.
- K. Hosokawa, K. Sato, N. Ichikawa and M. Maeda, Lab Chip, 2004, 4, 181 RSC.
- L. Qin, O. Vermesh, Q. Shi and J. R. Heath, Lab Chip, 2009, 9, 2016 RSC.
- I. K. Dimov, L. Basabe-Desmonts, J. L. Garcia-Cordero, B. M. Ross, Y. Park, A. J. Ricco and L. P. Lee, Lab Chip, 2011, 11, 845 RSC.
- R. M. Rocco, Landmark Papers in Clinical Chemistry., Elsevier, Amsterdam, 2006, vol. 52 Search PubMed.
- U. Y. Schaff and G. J. Sommer, Clin. Chem., 2011, 57, 753 CAS.
- M. Amasia and M. Madou, Bioanalysis, 2010, 2, 1701 CrossRef CAS PubMed.
- M. Grumann, J. Steigert, L. Riegger, I. Moser, B. Enderle, K. Riebeseel, G. Urban, R. Zengerle and J. Ducrée, Biomed. Microdevices, 2006, 8, 209 CrossRef CAS PubMed.
- J. Park, V. Sunkara, T.-H. Kim, H. Hwang and Y.-K. Cho, Anal. Chem., 2012, 84, 2133 CrossRef CAS PubMed.
- J. Steigert, T. Brenner, M. Grumann, L. Riegger, S. Lutz, R. Zengerle and J. Ducrée, Biomed. Microdevices, 2007, 9, 675 CrossRef CAS PubMed.
- S. Haeberle, T. Brenner, R. Zengerle and J. Ducrée, Lab Chip, 2006, 6, 776 RSC.
- J. Steigert, M. Grumann, T. Brenner, L. Riegger, J. Harter, R. Zengerle and J. Ducrée, Lab Chip, 2006, 6, 1040 RSC.
- J. Steigert, M. Grumann, T. Brenner, K. Mittenbuhler, T. Nann, J. Ruhe, I. Moser, S. Haeberle, L. Riegger and J. Riegler, J. Assoc. Lab. Autom., 2005, 10, 331 CrossRef CAS PubMed.
- B. S. Lee, Y. U. Lee, H.-S. Kim, T.-H. Kim, J. Park, J.-G. Lee, J. Kim, H. Kim, W. G. Lee and Y.-K. Cho, Lab Chip, 2011, 11, 70 RSC.
- B. S. Lee, J.-N. Lee, J.-M. Park, J.-G. Lee, S. Kim, Y.-K. Cho and C. Ko, Lab Chip, 2009, 9, 1548 RSC.
- J.-M. Park, Y.-K. Cho, B.-S. Lee, J.-G. Lee and C. Ko, Lab Chip, 2007, 7, 557 RSC.
- Y.-K. Cho, J.-G. Lee, J.-M. Park, B.-S. Lee, Y. Lee and C. Ko, Lab Chip, 2007, 7, 565 RSC.
- R. Gorkin, J. Park, J. Siegrist, M. Amasia, B. S. Lee, J.-M. Park, J. Kim, H. Kim, M. Madou and Y.-K. Cho, Lab Chip, 2010, 10, 1758 RSC.
- M. Madou, J. Zoval, G. Jia, H. Kido, J. Kim and N. Kim, Annu. Rev. Biomed. Eng., 2006, 8, 601 CrossRef CAS PubMed.
- C.-M. Cheng, A. W. Martinez, J. Gong, C. R. Mace, S. T. Phillips, E. Carrilho, K. A. Mirica and G. M. Whitesides, Angew. Chem., Int. Ed., 2010, 49, 4771 CrossRef CAS PubMed.
- Z. Nie, F. Deiss, X. Liu, O. Akbulut and G. M. Whitesides, Lab Chip, 2010, 10, 3163 RSC.
- E. Fu, B. Lutz, P. Kauffman and P. Yager, Lab Chip, 2010, 10, 918 RSC.
- B. R. Lutz, P. Trinh, C. Ball, E. Fu and P. Yager, Lab Chip, 2011, 11, 4274 RSC.
- E. Fu, P. Kauffman, B. Lutz and P. Yager, Sens. Actuators, B, 2010, 149, 325 CrossRef CAS PubMed.
- M. S. Khan, G. Thouas, W. Shen, G. Whyte and G. Garnier, Anal. Chem., 2010, 82, 4158 CrossRef CAS PubMed.
- W. Dungchai, O. Chailapakul and C. S. Henry, Anal. Chim. Acta, 2010, 674, 227 CrossRef CAS PubMed.
- E. M. Fenton, M. R. Mascarenas, G. P. López and S. S. Sibbett, ACS Appl. Mater. Interfaces, 2009, 1, 124 CAS.
- A. W. Martinez, S. T. Phillips, M. J. Butte and G. M. Whitesides, Angew. Chem., Int. Ed., 2007, 46, 1318 CrossRef CAS PubMed.
- A. W. Martinez, S. T. Phillips, E. Carrilho, S. W. Thomas, H. Sindi and G. M. Whitesides, Anal. Chem., 2008, 80, 3699 CrossRef CAS PubMed.
- A. K. Ellerbee, S. T. Phillips, A. C. Siegel, K. A. Mirica, A. W. Martinez, P. Striehl, N. Jain, M. Prentiss and G. M. Whitesides, Anal. Chem., 2009, 81, 8447 CrossRef CAS PubMed.
- X. Li, J. Tian and W. Shen, Anal. Bioanal. Chem., 2010, 396, 495 CrossRef CAS PubMed.
- J. L. Osborn, B. Lutz, E. Fu, P. Kauffman, D. Y. Stevens and P. Yager, Lab Chip, 2010, 10, 2659 RSC.
- X. Yang, O. Forouzan, T. P. Brown and S. S. Shevkoplyas, Lab Chip, 2012, 12, 274 RSC.
- S. J. Vella, P. Beattie, R. Cademartiri, A. Laromaine, A. W. Martinez, S. T. Phillips, K. A. Mirica and G. M. Whitesides, Anal. Chem., 2012, 84, 2883 CrossRef CAS PubMed.
- A. W. Martinez, S. T. Phillips and G. M. Whitesides, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 19606 CrossRef CAS PubMed.
- N. R. Pollock, J. P. Rolland, S. Kumar, P. D. Beattie, S. Jain, F. Noubary, V. L. Wong, R. A. Pohlmann, U. S. Ryan and G. M. Whitesides, Sci. Transl. Med., 2012, 4, 152ra129 CrossRef PubMed.
- A. K. Yetisen, M. S. Akram and C. R. Lowe, Lab Chip, 2013, 13, 2210 RSC.
- A. W. Martinez, S. T. Phillips, G. M. Whitesides and E. Carrilho, Anal. Chem., 2010, 82, 3 CrossRef CAS PubMed.
- W. Zhao and A. van der Berg, Lab Chip, 2008, 8, 1988 RSC.
- D. J. Raiten, S. Namasté, B. Brabin, J. Combs, Gerald, M. R. L'Abbe, E. Wasantwisut and I. Darnton-Hill, Am. J. Clin. Nutr., 2011, 94, 633S CrossRef PubMed.
- V. E. Hedrick, A. M. Dietrich, P. A. Estabrooks, J. Savla, E. Serrano and B. M. Davy, Nutr. J., 2012, 11, 109 CrossRef PubMed.
- C. J. Davis, D. Sowa, K. S. Keim, K. Kinnare and S. Peterson, JPEN, J. Parenter. Enteral Nutr., 2012, 36, 197 CrossRef CAS PubMed.
- M. P. Fuhrman, P. Charney and C. M. Mueller, J. Am. Diet. Assoc., 2004, 104, 1258 CrossRef CAS PubMed.
- H. Honda, A. R. Qureshi, O. Heimbürger, P. Barany, K. Wang, R. Pecoits-Filho, P. Stenvinkel and B. Lindholm, Am. J. Kidney Dis., 2006, 47, 139 CrossRef CAS PubMed.
- A. Shenkin, Clin. Chem., 2006, 52, 2177 CAS.
- W. Laiwattanapaisal, T. Songjaroen, T. Maturos, T. Lomas, A. Sappat and A. Tuantranont, Sensors, 2009, 9, 10066 CrossRef CAS PubMed.
- C.-J. Huang, C.-C. Lu, T.-Y. Lin, T.-C. Chou and G.-B. Lee, J. Micromech. Microeng., 2007, 17, 835 CrossRef CAS.
- C.-C. Lin, C.-C. Tseng, C.-J. Huang, J.-H. Wang and G.-B. Lee, Biomed. Microdevices, 2010, 12, 887 CrossRef CAS PubMed.
- W.-B. Lee, Y.-H. Chen, H.-I. Lin, S.-C. Shiesh and G.-B. Lee, Sens. Actuators, B, 2011, 157, 710 CrossRef CAS PubMed.
- Y.-N. Yang, H.-I. Lin, J.-H. Wang, S.-C. Shiesh and G.-B. Lee, Biosens. Bioelectron., 2009, 24, 3091 CrossRef CAS PubMed.
- M. Wolf, D. Juncker, B. Michel, P. Hunziker and E. Delamarche, Biosens. Bioelectron., 2004, 19, 1193 CrossRef CAS PubMed.
- M. C. Peoples and H. T. Karnes, Anal. Chem., 2008, 80, 3853 CrossRef CAS PubMed.
- S. Choi, A. Lajevardi-khosh and J. Chae, 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, 2011, 2223 CrossRef.
- G. Lee, I. Park, K. Kwon, T. Kwon, J. Seo, W.-J. Chang, H. Nam, G. S. Cha, M. H. Choi, D. S. Yoon and S. W. Lee, Biomed. Microdevices, 2012, 14, 375 CrossRef CAS PubMed.
- L. Gervais and E. Delamarche, Lab Chip, 2009, 9, 3330 RSC.
- K. Sun, N. Ramgir and S. Bhansali, Sens. Actuators, B, 2008, 133, 533 CrossRef CAS PubMed.
- A. Kumar, S. Aravamudhan, M. Gordic, S. Bhansali and S. S. Mohapatra, Biosens. Bioelectron., 2007, 22, 2138 CrossRef CAS PubMed.
- J. S. Mitchell, T. E. Lowe and J. R. Ingram, Analyst, 2009, 134, 380 RSC.
- K. S. Jaya Rao, S. G. Srikantia and C. Gopalan, Arch. Dis. Child., 1968, 43, 365 CrossRef CAS.
- M. Azahar Ali, S. Srivastava, P. R. Solanki, V. Varun Agrawal, R. John and B. D. Malhotra, Appl. Phys. Lett., 2012, 101, 084105 CrossRef.
- S. Aravamudhan, A. Kumar, S. Mohapatra and S. Bhansali, Biosens. Bioelectron., 2007, 22, 2289 CrossRef CAS PubMed.
- A. Wisitsoraat, P. Sritongkham, C. Karuwan, D. Phokharatkul, T. Maturos and A. Tuantranont, Biosens. Bioelectron., 2010, 26, 1514 CrossRef CAS PubMed.
- N. Ruecha, W. Siangproh and O. Chailapakul, Journal of Electronic Science and Technology, 2010, 8, 82 CAS.
- S. Aravamudhan, N. S. Ramgir and S. Bhansali, Sens. Actuators, B, 2007, 127, 29 CrossRef CAS PubMed.
- M. A. Ali, P. R. Solanki, M. K. Patel, H. Dhayani, V. V. Agrawal, R. John and B. D. Malhotra, Nanoscale, 2013, 5, 2883 RSC.
- J. Steigert, M. Grumann, M. Dube, W. Streule, L. Riegger, T. Brenner, P. Koltay, K. Mittmann, R. Zengerle and J. Ducrée, Sens. Actuators, A, 2006, 130–131, 228 CrossRef CAS PubMed.
- W. Schrott, M. Nebyla, L. Meisterová and M. Přibyl, Chem. Pap., 2010, 65, 246 CrossRef PubMed.
- E. P. Kartalov, D. H. Lin, D. T. Lee, W. F. Anderson, C. R. Taylor and A. Scherer, Electrophoresis, 2008, 29, 5010 CrossRef CAS PubMed.
- E. Kartalov, J. Zhong, A. Scherer, S. Quake, C. Taylor and W. French Anderson, BioTechniques, 2006, 40, 85 CrossRef CAS PubMed.
- P. Jothimuthu, R. A. Wilson, J. Herren, X. Pei, W. Kang, R. Daniels, H. Wong, F. Beyette, W. R. Heineman and I. Papautsky, Electroanalysis, 2013, 25, 401 CrossRef CAS.
- J. Hix, C. Martinez, I. Buchanan, J. Morgan, M. Tam and A. Shankar, The American Journal of Clinical Nutrition, 2004, 79, 93 CAS.
- A. Kaufmann, Anal. Bioanal. Chem., 2012, 403, 1233 CrossRef CAS PubMed.
- S. J. Lehotay and J. Hajšlová, TrAC, Trends Anal. Chem., 2002, 21, 686 CrossRef CAS.
- L. M. L. Nollet, F. Toldrá, Food analysis by HPLC, CRC Press, 2012 Search PubMed.
- R. A. Frazier and A. Papadopoulou, Electrophoresis, 2003, 24, 4095 CrossRef CAS PubMed.
- L. Asensio, I. González, T. García and R. Martín, Food Control, 2008, 19, 1 CrossRef CAS PubMed.
- Y. T. Atalay, S. Vermeir, D. Witters, N. Vergauwe, B. Verbruggen, P. Verboven, B. M. Nicolaï and J. Lammertyn, Trends Food Sci. Technol., 2011, 22, 386 CrossRef CAS PubMed.
- S. Neethirajan, I. Kobayashi, M. Nakajima, D. Wu, S. Nandagopal and F. Lin, Lab Chip, 2011, 11, 1574 RSC.
- Y.-F. Chen, L. Jiang, M. Mancuso, A. Jain, V. Oncescu and D. Erickson, Nanoscale, 2012, 4, 4839 RSC.
- A. Manz, N. Graber and H. M. Widmer, Sens. Actuators, B, 1990, 1, 244 CrossRef CAS.
- V. Dolník, S. Liu and S. Jovanovich, Electrophoresis, 2000, 21, 41 CrossRef.
- A. Escarpa, M. C. González, A. G. Crevillén and A. J. Blasco, Electrophoresis, 2007, 28, 1002 CrossRef CAS PubMed.
- A. Escarpa, M. C. González, M. A. López Gil, A. G. Crevillén, M. Hervás and M. García, Electrophoresis, 2008, 29, 4852 CrossRef CAS PubMed.
- G. Monti, L. De Napoli, P. Mainolfi, R. Barone, M. Guida, G. Marino and A. Amoresano, Anal. Chem., 2005, 77, 2587 CrossRef CAS PubMed.
- H. Ueno, J. Wang, N. Kaji, M. Tokeshi and Y. Baba, Journal of Separation Science, 2008, 31, 898 CrossRef CAS PubMed.
- A. G. Crevillén, A. J. Blasco, M. C. González and A. Escarpa, Electrophoresis, 2006, 27, 5110 CrossRef PubMed.
- V. Blazek and R. A. Caldwell, Int. J. Food Sci. Technol., 2009, 44, 2127 CrossRef CAS.
- V. K. S. Hsiao, J. R. Waldeisen, Y. Zheng, P. F. Lloyd, T. J. Bunning and T. J. Huang, J. Mater. Chem., 2007, 17, 4896 RSC.
- S. Yang, F. Guo, B. Kiraly, X. Mao, M. Lu, K. W. Leong and T. J. Huang, Lab Chip, 2012, 12, 2097 RSC.
- S. Yang, M. I. Lapsley, B. Cao, C. Zhao, Y. Zhao, Q. Hao, B. Kiraly, J. Scott, W. Li, L. Wang, Y. Lei and T. J. Huang, Adv. Funct. Mater., 2013, 23, 720 CrossRef CAS.
- B. Pérez-López and A. Merkoçi, Trends Food Sci. Technol., 2011, 22, 625 CrossRef PubMed.
- A. G. Crevillén, M. Avila, M. Pumera, M. C. González and A. Escarpa, Anal. Chem., 2007, 79, 7408 CrossRef PubMed.
- F. Fernández, K. Hegnerová, M. Piliarik, F. Sanchez-Baeza, J. Homola and M.-P. Marco, Biosens. Bioelectron., 2010, 26, 1231 CrossRef PubMed.
- Y. Gao, F. Guo, S. Gokavi, A. Chow, Q. Sheng and M. Guo, Food Chem., 2008, 110, 769 CrossRef CAS PubMed.
- Y. B. Zheng, T. J. Huang, A. Y. Desai, S. J. Wang, L. K. Tan, H. Gao and A. C. H. Huan, Appl. Phys. Lett., 2007, 90, 183117 CrossRef.
- Y. B. Zheng, L. Jensen, W. Yan, T. R. Walker, B. K. Juluri, L. Jensen and T. J. Huang, J. Phys. Chem. C, 2009, 113, 7019 CAS.
- Y. B. Zheng, B. Kiraly, P. S. Weiss and T. J. Huang, Nanomedicine, 2012, 7, 751 CrossRef CAS PubMed.
- Y. J. Liu, Q. Hao, J. S. T. Smalley, J. Liou, I. C. Khoo and T. J. Huang, Appl. Phys. Lett., 2010, 97, 091101 CrossRef.
- B. K. Juluri, Y. B. Zheng, D. Ahmed, L. Jensen and T. J. Huang, J. Phys. Chem. C, 2008, 112, 7309 CAS.
- C. Situ, M. H. Mooney, C. T. Elliott and J. Buijs, TrAC, Trends Anal. Chem., 2010, 29, 1305 CrossRef CAS PubMed.
- S. Vyawahare, A. D. Griffiths and C. A. Merten, Chem. Biol., 2010, 17, 1052 CrossRef CAS PubMed.
- W. Laiwattanapaisal, J. Yakovleva, M. Bengtsson, T. Laurell, S. Wiyakrutta, V. Meevootisom, O. Chailapakul and J. Emnéus, Biomicrofluidics, 2009, 3, 14104 CrossRef CAS PubMed.
- M. Kamruzzaman, A.-M. Alam, S. H. Lee and T. D. Dang, Sens. Actuators, B, 2013, 185, 301 CrossRef CAS PubMed.
- K. S. Lok, S. Z. binte Abdul Muttalib, P. P. F. Lee, Y. C. Kwok and N.-T. Nguyen, Lab Chip, 2012, 12, 2353 RSC.
- M. Kamruzzaman, A.-M. Alam, K. M. Kim, S. H. Lee, Y. H. Kim, A. N. M. H. Kabir, G.-M. Kim and T. D. Dang, Biomed. Microdevices, 2013, 15, 195 CrossRef CAS PubMed.
- D. Hoegger, P. Morier, C. Vollet, D. Heini, F. Reymond and J. S. Rossier, Anal. Bioanal. Chem., 2007, 387, 267 CrossRef CAS PubMed.
- L. Cai, Y. Wu, C. Xu and Z. Chen, J. Chem. Educ., 2013, 90, 232 CrossRef CAS.
- J. L. Garcia-Cordero, L. M. Barrett, R. O'Kennedy and A. J. Ricco, Biomed. Microdevices, 2010, 12, 1051 CrossRef CAS PubMed.
- H. Hwang, Y. Kim, J. Cho, J. Lee, M.-S. Choi and Y.-K. Cho, Anal. Chem., 2013, 85, 2954 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2013 |
