 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrogel-coated microfluidic channels for cardiomyocyte culture†
Nasim
Annabi
abc,
Šeila
Selimović‡
ab,
Juan Pablo
Acevedo Cox‡
d,
João
Ribas
abef,
Mohsen
Afshar Bakooshli
ab,
Déborah
Heintze
abg,
Anthony S.
Weiss
hij,
Donald
Cropek
k and
Ali
Khademhosseini
*abc
aCenter for Biomedical Engineering, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Cambridge, Massachusetts 02139, USA. E-mail: alik@rics.bwh.harvard.edu
bHarvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA
cWyss Institute for Biologically Inspired Engineering, Harvard University, Boston, Massachusetts 02115, USA
dFaculty of Medicine and Faculty of Engineering and Applied Science, University of the Andes, Santiago 7620001, Chile
ePhD Programme in Experimental Biology and Biomedicine, CNC-Center for Neuroscience and Cell Biology and Institute for Interdisciplinary Research (IIIUC), University of Coimbra, 3030-789 Coimbra, Portugal
fBiocant - Center of Innovation in Biotechnology, 3060-197 Cantanhede, Portugal
gInstitute of Bioengineering and School of Life Sciences, Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015, Lausanne, Switzerland
hSchool of Molecular Bioscience, University of Sydney, 2006, Australia
iBosch Institute, University of Sydney, 2006, Australia
jCharles Perkins Centre, University of Sydney, 2006, Australia
kUS Army Corps of Engineers Construction Engineering Research Laboratory, Champaign, IL 61822, USA
First published on 10th May 2013
Abstract
The research areas of tissue engineering and drug development have displayed increased interest in organ-on-a-chip studies, in which physiologically or pathologically relevant tissues can be engineered to test pharmaceutical candidates. Microfluidic technologies enable the control of the cellular microenvironment for these applications through the topography, size, and elastic properties of the microscale cell culture environment, while delivering nutrients and chemical cues to the cells through continuous media perfusion. Traditional materials used in the fabrication of microfluidic devices, such as poly(dimethylsiloxane) (PDMS), offer high fidelity and high feature resolution, but do not facilitate cell attachment. To overcome this challenge, we have developed a method for coating microfluidic channels inside a closed PDMS device with a cell-compatible hydrogel layer. We have synthesized photocrosslinkable gelatin and tropoelastin-based hydrogel solutions that were used to coat the surfaces under continuous flow inside 50 μm wide, straight microfluidic channels to generate a hydrogel layer on the channel walls. Our observation of primary cardiomyocytes seeded on these hydrogel layers showed preferred attachment as well as higher spontaneous beating rates on tropoelastin coatings compared to gelatin. In addition, cellular attachment, alignment and beating were stronger on 5% (w/v) than on 10% (w/v) hydrogel-coated channels. Our results demonstrate that cardiomyocytes respond favorably to the elastic, soft tropoelastin culture substrates, indicating that tropoelastin-based hydrogels may be a suitable coating choice for some organ-on-a-chip applications. We anticipate that the proposed hydrogel coating method and tropoelastin as a cell culture substrate may be useful for the generation of elastic tissues, e.g. blood vessels, using microfluidic approaches.
Introduction
Incorporating living cells into microfabricated devices has become a focus of interest for applications in tissue engineering,1–4 diagnostics5–7 and drug screening.8–12 Reducing the cell-based experimental platform from the standard well-plate devices to a microfluidic device has several advantages, including a reduction in reagent amount and experiment duration, and therefore a cost reduction.13,14 Moreover, the number of cells required for a microfluidic experiment is significantly smaller than for a well-plate setup. This is especially important for studies relying on primary cells extracted from sacrificed animals. Additionally, microfluidic devices provide spatially and temporally well-controlled microenvironments for cell culture.3,15In the field of microfluidics, poly(dimethylsiloxane) (PDMS) is often the material of choice.16 It is largely transparent to visible and UV light, permeable to air and water, but not to polar and large molecules. Most importantly, it is compatible with cells when fully cured. However, untreated PDMS is hydrophobic and therefore not suitable for direct cell attachment and proliferation. Coating PDMS with proteins that attach to hydrophobic surfaces, such as fibronectin, is one solution to this challenge. Also, the PDMS surface can temporarily be rendered hydrophilic through exposure to oxygen plasma,17 or permanently using sol–gel chemistry.18 However, these methods are cumbersome and do not allow for tuning of the material stiffness.
Tissue engineering scaffolds provide temporary extracellular matrix (ECM) environments to support cell growth as well as to regulate cellular responses during tissue formation. Hydrogels are attractive scaffolds for tissue engineering applications, as they resemble the characteristics of natural ECM.19 Hydrogels have been fabricated in a variety of planar and three dimensional (3D) shapes,20–22 using different types of ECM components such as collagen,23 gelatin,24,25 hyaluronic acid,26,27 and elastin.28,29 Gelatin is the denatured form of collagen and can be easily isolated from animals. Although it is a denatured protein, it contains cell-binding sites, which can facilitate cellular attachment and growth. Tropoelastin is a natural, resilience-imparting protein found in all elastic human tissues and has been used as a biomaterial for engineering elastic tissue constructs.30 We have previously shown that modification of these two ECM components with methacrylate groups renders them photocrosslinkable, such that they can be patterned via UV light into a variety of geometries.25,31 We have demonstrated that these photocrosslinkable ECM gels can support cellular growth both in 2D and 3D environments.20,25,31
In this study, we developed a new technique to coat a PDMS-based microfluidic device with methacrylated tropoelastin (MeTro) and methacrylated gelatin (GelMA) hydrogels to facilitate cardiomyocyte (CM) attachment and organization inside the channels. Using a single illumination intensity and defined crosslinking time, our coating technique enabled tuning of the coating thickness by adjusting the injection flow rate of the prepolymer solution during UV crosslinking. We investigated the effect of hydrogel concentration, crosslinked inside the channels, on CM attachment, spreading, alignment and beating behaviors. The hydrogel coated microfluidic device developed in this study has the potential to be used for engineering of various organ-on-a-chip platforms.
Methods and materials
Device fabrication protocol
The microfluidic platform used in this study contained a total of 15 connected parallel fluidic channels, which shared a single input line and a single output line. Thus, all channels were supplied with the same solutions, offering experimental data with a redundancy of n = 15. The channels were designed to be 1 cm long, 50 μm wide and 150 μm tall, although there were minor variations in the channel height (±10 μm) between different devices, stemming from differences in the master molds. The devices were fabricated using standard photo- and soft lithography protocols. Namely, the master silicon wafer (Silicon Sense, Inc., Nashua, NH) was patterned with negative photoresist (SU8-2050, MicroChem Corp., Newton, MA) by spin-coating at 1500 rpm for 30 s, followed by a soft bake (5 min at 65 °C and 18 min at 95 °C) and UV exposure at 260 mJ cm−2. These resin-coated wafers were post-exposure baked (4 min at 65 °C and 9 min at 95 °C) and developed using propylene glycol methyl ether acetate (PGMEA, 484431, Sigma-Aldrich, St. Louis, MO), followed by a 30 min long hardening bake at 180 °C. The fluidic channels were cast in 4 mm thick PDMS (Sylgard 184, Dow Corning, Midland, MI) and sealed with a 30 μm thin spin-coated PDMS layer (550 rpm for 18 s and 1100 rpm for 60 s) using oxygen plasma (1000 Watt exposure for 20 s, Harrick Plasma, Ithaca, NY). Prior to bonding, both PDMS layers were individually baked for 60 min at 80 °C. The assembled devices were sterilized with UV light prior to the introduction of cells.GelMA synthesis
GelMA was synthesized according to the protocol previously described.25 Briefly, gelatin from porcine skin (G2500, Sigma-Aldrich) was dissolved at 10% (w/v) in sterile Dulbecco's Phosphate Buffer Saline (DPBS, 14190-144, Life Technologies, Grand Island, NY). The suspension was mixed for 1 h at 60 °C and 200 rpm. In order to methacrylate the gelatin, 8% (v/v) methacrylic anhydride (276685, Sigma-Aldrich) was slowly added to the protein solution. After 3 h at 60 °C, the solution was diluted in DPBS to end the reaction and dialyzed against deionized water for 7 days at 40 °C and 500 rpm. Finally, the solution was filtered with a 20 μm filter and lyophilized to obtain a GelMA foam.MeTro synthesis
Tropoelastin isoform SHELΔ26A (Synthetic Human Elastin without domain 26A) corresponding to amino acid residues 27–724 of GenBank entry AAC98394 was purified from bacteria as previously described.32 The protein was methacrylated by the addition of 8% (v/v) methacrylic anhydride to a 10% (w/v) tropoelastin solution in DPBS and reacted for 12 h at 4 °C. The solution was then diluted and dialyzed (Slide-A-Lyzer MINI, 3.5 K MWCO) against distilled water at 4 °C for 48 h and lyophilized to yield MeTro.Channel coating
GelMA- and MeTro-based hydrogels were used independently to coat the PDMS-based microchannels and to provide an attachment surface for CMs. To coat the PDMS-based microfluidic channels, various concentrations of hydrogel prepolymer solutions (5, 8, and 10% (w/v)) containing photoinitiator (0.5% (w/v) Irgacure 2959, BASF, Ludwigshafen, Germany) in DPBS were introduced into the devices and crosslinked under UV light. Fluorescent dye (10% (v/v) of fluorescein isothiocyanate (FITC)-conjugated Dextran 10 kDa, Sigma-Aldrich, St. Louis, MO) was added to the bulk prepolymer solution prior to UV-exposure to enable visual detection and measurement of the hydrogel coating thickness, but was omitted in cell experiments. Each device was exposed to UV light (OmniCure 2000, Mississauga, ON, Canada) for 3 min at 14.6 mW cm−2 (GelMA) and 6.90 mW cm−2 (MeTro). During UV exposure, the hydrogel solution was perfused at a constant flow rate (ranging from 0 μl h−1 to 1200 μl h−1) through the device. The Reynolds number (Re) at the highest flow rate was 0.025, indicating laminar flow. For cell experiments, we used a flow rate of 900 μl h−1 for GelMA and 1020 μl h−1 for MeTro to form a thin hydrogel coating of uniform thickness. After the crosslinking step, each device was washed for 5 min with DPBS in order to remove any uncrosslinked prepolymer solution and residual photoinitiator that might potentially be harmful to cells. All devices were stored overnight in DPBS at 4 °C to prevent drying and cracking of the hydrogel. Devices were then cut with a razor blade to obtain images of the channel cross-sections or they were seeded with cells (Fig. 1). The thickness of the fluorescently labelled hydrogel layers in each microfluidic channel was measured using ImageJ software (http://rsbweb.nih.gov/ij/) and averaged.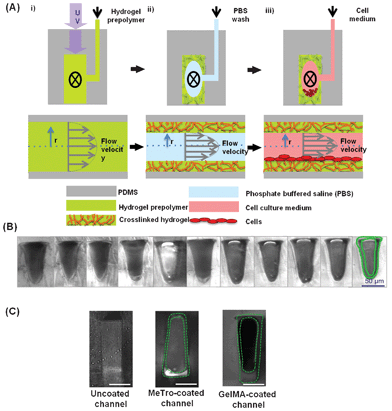 | ||
| Fig. 1 Schematic of the coating procedure (A): i) A hydrogel prepolymer is flowed through the device while being exposed to UV light for crosslinking. ii) The uncrosslinked prepolymer is washed with PBS, while the crosslinked hydrogel layer remains inside the channel and coats the PDMS channel walls. iii) The device can subsequently be loaded with cells and perfused without removing the hydrogel layer. The top figures show the cross-section of a single microfluidic channel, perpendicular to the direction of the flow. The bottom figures also show the channel cross-section, along the direction of the flow. (B) A series of phase contrast images showing microfluidic channels from a single device coated with 5% (w/v) GelMA after a 6-day long perfusion with culture medium. The hydrogel layer is outlined in green in the far right image and can be seen in the same position in the neighboring photographs. (C) Representative phase contrast images from an uncoated channel, 5% MeTro-coated channel (1020 μl h−1 flow rate), and 5% (w/v) GelMA-coated channel (900 μl h−1) (scale bar: 50 μm). | ||
Cell isolation and culture
Primary CMs were obtained from 2-day-old neonatal Sprague-Dawley rats according to the protocol approved by the Institute's Committee on Animal Care. Briefly, the hearts of Sprague-Dawley neonatal pups were excised after inducing narcosis with compressed CO2 gas. The isolated hearts were then incubated in a 0.06% (w/v) solution of trypsin in Hank's Balanced Salt Solution (HBSS, 14025-076) buffer at 4 °C for 16 h on a shaker to digest the tissues. The heart tissues were subjected to a series of digestions in 0.1% (w/v) collagenase type II (LS004176, Worthington, Lakewood, NJ) solution in HBSS (10 min shaking at 37 °C for each digestion). The supernatant for the first digestion was discarded, and the cell suspensions from the subsequent two to three digestions were collected, centrifuged at 1200 rpm, and resuspended in Dulbecco's Modified Eagle Medium (DMEM, 11965-092) supplemented with 10% fetal bovine serum (FBS), 100 units ml penicillin-streptomycin, and 2 mM L-glutamine. The cell suspension was pre-plated for a 1 h period to enrich for CMs. Namely, the attached cells were cardiac fibroblasts and the unattached ones were CMs. Then, the unattached CM cells were used for seeding inside the devices coated with MeTro and GelMA. Unless noted otherwise, all cell isolation and culture reagents were obtained from Life Technologies, Grand Island, NY.Cell loading
Prior to cell loading, all devices were filled with culture media and kept in a humidified cell culture incubator for 4 h (37 °C, 5% CO2) to allow the proteins in the media to diffuse into the hydrogel layer. The cell suspension was then manually loaded into the device at a concentration of 2 × 108/ml until cells were uniformly distributed along all channels. The devices were kept for 3 h undisturbed in the culture incubator to allow the cells to attach to the hydrogel layer. After 3 h, the devices were perfused with culture media inside the incubator for 6 days at a flow rate of 20 μl h−1.CM attachment and alignment
Cell attachment on the surfaces of microchannels coated with various concentrations of MeTro and GelMA was assessed 3 h after CM seeding inside the devices. The effect of media perfusion flow rate on cell attachment was investigated by applying perfusion flow at 40 μl h−1 for 1 min and then increasing the flow rate to 100 μl h−1. A video sequence was recorded during this flow rate change. The cell displacement on 10% (w/v) MeTro- and GelMA-coated devices was then calculated by comparing the cell movement in the first frame (A1) and the last frame (A2) of the video (overlay) using ImageJ software (Supplementary Fig. 1).Rhodamine-phalloidin (Alexa-Fluor 594 Phalloidin, A12381, Life Technologies, Grand Island, NY) and 4′,6-diamidino-2-phenylindole (DAPI, D8417, Sigma-Aldrich) staining was used to quantify the cellular attachment and alignment on the surfaces of microchannels coated with GelMA and MeTro after 3 h and on day 6 of culture. For DAPI staining, the microfluidic devices with the cell-seeded gels were fixed in 4% paraformaldehyde (15710, Electron Microscopy Sciences, Hatfield, PA) solution in DPBS for 30 min. To stain the cell nuclei, the devices were then incubated in a 0.1% (v/v) DAPI solution in PBS for 10 min at 37 °C. The devices were perfused with DPBS and visualized with an inverted laser scanning confocal microscope (Leica SP5 XMP, Germany). ImageJ software (http://rsbweb.nih.gov/ij/) was used to count the DAPI stained nuclei and compare the cell attachment on the surfaces of channels coated with various concentrations of GelMA and MeTro. Three images from three individual samples for each sample type (MeTro and GelMA) were analyzed.
F-actin staining was used to quantify cellular alignment inside the microchannels coated with MeTro and GelMA. Cell-seeded channels were first fixed in 4% paraformaldehyde in DPBS for 30 min. The cells were then permeabilized in a 0.1% (w/v) Triton X-100 solution in DPBS for 20 min and blocked in 1% (w/v) bovine serum albumin (BSA) for 1 h. The devices were incubated in a solution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 ratio of Alexa Fluor-594 phalloidin in 0.1% BSA for 45 min at room temperature to stain the actin cytoskeleton. The devices were perfused with PBS and visualized with an inverted fluorescence microscope. Cellular alignment was determined using fluorescence images according to the previously described methods.33
40 ratio of Alexa Fluor-594 phalloidin in 0.1% BSA for 45 min at room temperature to stain the actin cytoskeleton. The devices were perfused with PBS and visualized with an inverted fluorescence microscope. Cellular alignment was determined using fluorescence images according to the previously described methods.33
Immunostaining
The expression of CM proteins inside the channels coated with 5% (w/v) hydrogels was assessed by immunostaining for troponin I, connexin 43, and sarcomeric α-actinin on day 6 of culture. In the immunostaining procedure, the devices were first washed with DPBS and the cells fixed in 4% paraformaldehyde solution for 30 min. The detergent Triton X-100 0.1%, (T9284, Sigma-Aldrich) followed by 10% goat serum in DPBS were introduced into the devices and incubated for 30 min. The device was then left overnight at 4 °C with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 dilution of the primary antibodies (rabbit-anti-connexin 43, mouse-anti-troponin, and mouse-anti-sarcomeric α-actinin) in goat serum, without being rinsed first with DPBS. Following a DPBS wash, secondary antibodies (Alexa Fluor 488 goat-anti-mouse for troponin I and sarcomeric α-actinin, and Alexa Fluor 594 goat-anti-rabbit for connexin 43) were introduced into the devices at a 1
200 dilution of the primary antibodies (rabbit-anti-connexin 43, mouse-anti-troponin, and mouse-anti-sarcomeric α-actinin) in goat serum, without being rinsed first with DPBS. Following a DPBS wash, secondary antibodies (Alexa Fluor 488 goat-anti-mouse for troponin I and sarcomeric α-actinin, and Alexa Fluor 594 goat-anti-rabbit for connexin 43) were introduced into the devices at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 dilution in goat serum for 2 h. After several washes with DPBS, we introduced a solution of 4′,6-diamidino-2-phenylindole dihydrochloride in DPBS (0.2% (v/v)) into the device, incubated it for 5–7 min at 37 °C and then rinsed it with DPBS.
200 dilution in goat serum for 2 h. After several washes with DPBS, we introduced a solution of 4′,6-diamidino-2-phenylindole dihydrochloride in DPBS (0.2% (v/v)) into the device, incubated it for 5–7 min at 37 °C and then rinsed it with DPBS.
Characterization of CM beating inside microchannels
CM beating behaviors within the channels coated with various concentrations of MeTro and GelMA gels were assessed using movies recorded daily for 6 days with a video camera (Sony XCD-X710) attached to an inverted optical microscope (Nikon, Eclipse TE 200U, Japan), at 10× magnification. To maintain the samples at 37 °C during video recording, the microscope was equipped with a temperature controller. To quantify the beat frequency (number of beats/min), videos taken from at least 3 different channels of 2 individual devices coated with MeTro and GelMA were analyzed using a custom-written Matlab routine. The effect of prepolymer concentration on the spontaneous contractions of CMs was determined by using various polymer solution concentrations (5, 8, and 10% (w/v)).Results and discussion
Channel coating
In this paper we present a new method for coating microfluidic channels with photocrosslinkable hydrogels. Our approach provides an adequate support for cell adhesion and proliferation as well as control of the biochemical and mechanical microenvironment for cell culture. It exploits UV light and properties of laminar flow inside microfluidic channels to create a solidified layer of a continuously flowing photocrosslinkable prepolymer along the channel walls. Here, we assume a parabolic flow profile as a characteristic of laminar flow inside straight rectangular microchannels, and a no-slip boundary condition.34,35 Then, fluid packets near the channel walls travel at a lower velocity than those in the center of the channel, such that their residence time is long enough for progressive polymerization and thickening into solid material under continuous and homogeneous UV irradiation (Fig. 1).In this study, the microfabricated device contained a total of 15 connected parallel fluidic channels, which shared a single input line and a single output line. This design was advantageous in terms of statistical sampling, allowing a large number of replicates in one experiment. In addition, this complex device constituted a more challenging setting to prove the new coating method compared to a single channel device. We used recombinant human tropoelastin and gelatin extracted from bovine skin to synthesize photocrosslinkable MeTro and GelMA gels, respectively. These biological ECM components, harboring covalently attached methacrylate functional groups, can generate crosslinked hydrogel networks upon UV irradiation in the presence of a photoinitiator. At relatively low photoinitiator concentrations (e.g. 0.5% (w/v) Irgacure 2959, BASF, Ludwigshafen, Germany), cell viability in both 2D and 3D culture is not affected negatively, especially when any residual molecules are washed away.25
We hypothesized that the prepolymer solution on the channel walls would progressively polymerize in response to the reduced local flow velocity. This expectation was corroborated by the observation that the amount of crosslinked hydrogel present inside a channel, that is, hydrogel thickness, decreased with higher perfusion rates and shorter residence times inside the device during an applied flow. Here, the term residence time refers to the total time a prepolymer fluid packet spends inside the device: from the time it enters the microfluidic structure to the time when it exits. In addition, when no flow was applied during the crosslinking step, all prepolymer present inside the microfluidic channels gelled, thereby permanently clogging the channels. This method enabled homogeneous coating of PDMS-based channels with either hydrogel. There were no significant differences in the coating thickness of MeTro and GelMA hydrogels at low flow rates (Fig. 2), but at comparatively high flow rates (>700 μl h−1) the MeTro thickness was consistently higher. To achieve a similar thickness of the GelMA and MeTro hydrogels for our cell experiments, we chose a GelMA flow rate of 900 μl h−1 and a MeTro flow rate of 1020 μl h−1. At these conditions (and 5% (w/v) of either hydrogel) roughly 15–20 μm thick coatings could be formed, which ensured a sufficiently soft surface for cellular attachment without blocking large parts of the microchannels.
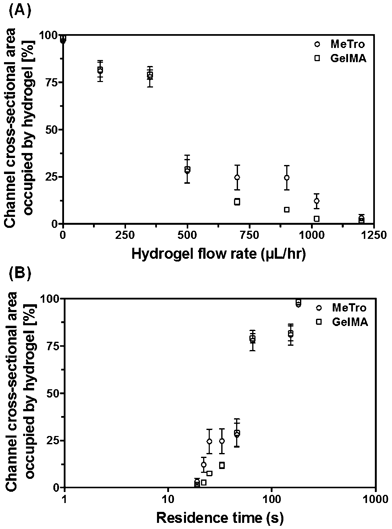 | ||
| Fig. 2 Average amount of crosslinked hydrogel occupying the microfluidic channels, expressed in % of the channel cross-section, as a function of the hydrogel perfusion flow rate (A) and the total residence time inside the device during an applied flow (B). The UV intensities were 14.6 mW cm−2 (for 5% (w/v) GelMA) and 6.90 mW cm−2 (for 5% (w/v) MeTro), and the UV exposure lasted 3 min. The photoinitiator concentration was 0.5% (w/v) for both hydrogels. The error bars depict the standard error. | ||
It is noteworthy that neither MeTro nor GelMA bonds with PDMS during the crosslinking process, as evidenced by peeling of planar hydrogel sheets from PDMS. We believe that this was because the hydrogels are hydrophilic and PDMS is hydrophobic. Instead, the crosslinked hydrogels adopt a tubular shape inside the microfluidic channels, which does not degrade after continuous perfusion with media for 6 days (Fig. 1B). Therefore, PDMS need not be hydrophilized, e.g. via oxygen plasma, nor pretreated with reagents such as 3-(Trimethoxysilyl)propyl methacrylate, which promote covalent bonding of MeTro and GelMA to a substrate.
CM attachment and alignment
After standardizing the coating protocol (at a set UV intensity), we used neonatal rat CMs as model cells to test the effect of coating method on cell attachment and proliferation. To study the effect of hydrogel concentration on cellular attachment inside the microchannels, the devices were coated with various concentrations of MeTro and GelMA (5, 8, and 10% (w/v)). Culture media was then perfused inside coated microchannels 3 h after cell seeding. Cell attachment to the channel walls was assessed using phase contrast and DAPI-stained fluorescence images (Fig. 3A–C). In general, more cells were observed on MeTro-coated microchannels compared to the channels coated with GelMA. Following media perfusion, most of the cells detached from the channel walls coated with GelMA, however, CMs cultured inside the MeTro-coated channels continued to adhere to the substrate (supporting videos SV1 and SV2, ESI†).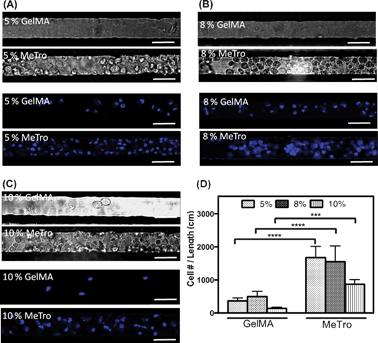 | ||
| Fig. 3 CMs attachment inside 50 μm wide microfluidic channels coated with GelMA and MeTro, using (A) 5, (B) 8, and (C) 10% (w/v) prepolymer solutions, 3 h after seeding. Phase contrast images are shown in top panels and fluorescence images from 4′,6-diamidino-2-phenylindole (DAPI)-stained cell nuclei are shown in bottom panels (scale bar = 50 μm). (D) Cell densities, defined as the number of DAPI stained nuclei per cm of coated microchannels with MeTro and GelMA at varying prepolymer concentrations. Error bars represent the SD of measurements performed on 5 samples (***p < 0.001). | ||
In our previous study, we observed a similar behavior for CM-seeded GelMA and MeTro hydrogels cultured in 24 well-plates.36 CM attachment to MeTro-coated microchannels can be facilitated by both the presence of cell-interactive amino acid sequences in tropoelastin37 and the higher elasticity of the MeTro gel compared to GelMA.31 We have shown that the elastic modulus of MeTro hydrogels varied from 3 to 15 kPa depending on the polymer concentration.31 However, the elastic modulus of GelMA hydrogels was less than 3 kPa at the highest concentration. The elastic modulus of rat CMs is reported to be 30 kPa,38 which is closer to the elastic modulus of the MeTro gel compared to that of GelMA. In addition, cardiac tissues have been shown to rely on matrix elasticity to preserve cell viability, organization and tissue function.39 MeTro gels are highly elastic with extensibility of up to 400%, which is substantially more than the extensibility of GelMA gels (<100%).31
We also investigated the effect of prepolymer concentration on the CM attachment inside the microchannels coated with GelMA and MeTro. As shown in Fig. 3D, increasing the prepolymer concentration had no significant effects on cell attachment. In general, fewer cells were attached on the surfaces of microchannels coated with 10% (w/v) polymer solution. This could be due to the higher crosslinking density and subsequently increased stiffness and decreased elasticity of these hydrogel layers. We suggest that the CMs prefer more elastic gels to attach and spread (e.g. 5% (w/v) compared to 10% (w/v)).31
Cellular alignment inside the microchannels was investigated by using F-actin stained fluorescence images of the devices coated with MeTro (Fig. 4A–C) and GelMA (Fig. 4G–I) on day 6 of culture, according to a previously detailed procedure.33 In our previous study, we have shown that cells encapsulated in GelMA gels had adopted a preferred orientation inside narrow channels (50 μm wide).20 Therefore, in this study, we used devices containing 50 μm wide microchannels to facilitate the CM alignment and formation of cardiac myofibers. As shown in Fig. 4, CMs elongated along the flow direction and created aligned cardiac fibers inside the microchannels. Histograms of the F-actin alignment inside the channels coated with MeTro and GelMA are shown in Fig. 4D–F and Fig. 4J–L, respectively. CM alignment was slightly higher in MeTro-coated channels than in those coated with GelMA (Fig. 4M). In addition, the GelMA concentration had no significant effect on cellular alignment within the channels. However, the strongest CM alignment was obtained within the 5% (w/v) MeTro-coated microchannels. The cardiac fibers formed inside the 50 μm coated channels in our study mimic the morphology of native myofibers with 50 μm width.40,41 We emphasize that although both MeTro- and GelMA-coated channels were lined with cells by day 6, the initial cell attachment was stronger on MeTro than on GelMA.
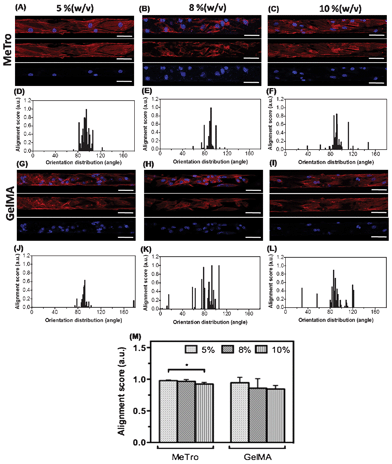 | ||
| Fig. 4 CM elongation and alignment inside the microchannels coated with MeTro and GelMA on day 6 of culture. Representative images of Rhodamine-labelled phalloidin/DAPI stained cells seeded on (A–C) MeTro-coated, and (G–I) GelMA-coated microchannels (scale bar = 50 μm). Histograms of the angle distribution of cells inside channels coated with (D–F) MeTro and (J–L) GelMA (90° represents cells parallel to channel while 0°/180° represent perpendicular). (M) Alignment scores for different concentrations of MeTro and GelMA. Error bars represent the SD of measurements performed on 3 samples (*p < 0.05). | ||
Immunohistochemical analysis
The expression of cardiac differentiation markers, including troponin I, sarcomeric α-actinin, and connexin 43, inside the microchannels coated with MeTro and GelMA was evaluated on day 6 of culture. As shown in Fig. 5A and B, both GelMA- and MeTro-coated microchannels promoted the expression of troponin I. Hydrogel coating of the devices facilitated the formation of a pervasive, well-developed, contractile apparatus inside the channels. In addition, it was found that the expressions of connexin 43 and Sarcomeric α-actinin were stronger inside the MeTro-coated channels than inside GelMA-coated ones. The presence of a MeTro layer inside the PDMS device facilitated the formation of elongated, well-defined, cross-striated sarcomeric structures (Fig. 5C) that resemble those of native adult rat ventricular myocardium.42 In contrast, scattered and less organized sarcomeres appeared inside GelMA-coated channels (Fig. 4D). We also observed patches of connexin 43 staining in different channels coated with 5% (w/v) MeTro gel, confirming the presence of well-developed intercalated disk and gap junctions between myocytes. However, in GelMA-coated device, connexin 43 was sparse and diffuse throughout the channels.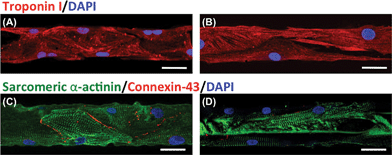 | ||
| Fig. 5 Confocal microscopy images showing immunostaining of CM markers inside microchannels coated with (A, C) 5% (w/v) MeTro and (B, D) 5% (w/v) GelMA on day 6 of culture. Hydrogels stained for (A, B) troponin (red)/nuclei (blue) and (C, D) sarcomeric α-actinin (green)/connexin-43 (red)/nuclei (blue) (scale bar = 50 μm). | ||
In native heart tissue, the presence of gap junctions on the lateral surfaces of the CMs plays an important role for cellular linking and electrical coupling.43 The well-developed networks of gap junctions and sarcomeres inside the MeTro-coated channels mimic the structures of native myocardium. Our data are in agreement with previous studies, in which the ECM proteins were shown to promote CM spreading and maturation due to their ability to provide appropriate cellular cues.44,45
Beating characteristics
The beating behavior of CMs inside microchannels coated with MeTro and GelMA was investigated during 6 days of culture. CMs seeded in MeTro-coated microchannels, using various polymer concentrations, displayed synchronous contractions as early as day 4 of culture (Fig. 6A–C). In contrast, contractions of CMs inside GelMA-coated channels were weaker and less synchronized compared to MeTro-coated ones (supporting videos SV3 and SV4, ESI†).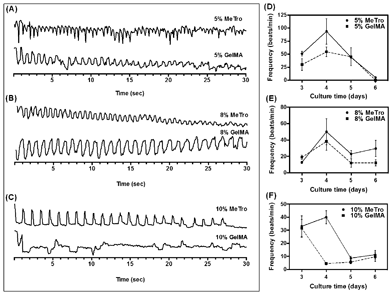 | ||
| Fig. 6 Beating behavior of CMs seeded inside microchannels coated with MeTro and GelMA using (A) 5, (B) 8, (C) 10%(w/v) prepolymer solutions on day 4 of culture. Spontaneous beating frequency of CMs seeded inside microchannels coated with (D) 5, (E) 8, and (F) 10%(w/v) gels over 6 days of culture. | ||
The beating rate was quantified daily for 6 days of culture for both MeTro- and GelMA-coated devices and various concentrations of prepolymer solution. As shown in Fig. 6D–F, the beating rate varied between 4–124 beats/min for MeTro-coated devices, depending on polymer concentration and culture time. For all concentrations of MeTro, the highest beating rate was observed on day 4 of culture, after which it started to decrease. A similar trend was observed for GelMA-coated channels, but the beating rate here varied between 2–75 beats/min, which was lower than that of MeTro-coated channels. These results are consistent with previous studies, in which neonatal rat CMs cultured on hyaluronic acid or Matrigel substrates exhibited spontaneous beating, but whose beating rates decreases with increasing culture time (within 7 days of culture).46,47
The CM beating rate was generally higher in MeTro-coated devices than GelMA-coated ones (e.g. 138 ± 38 beats/min compared to 54 ± 18 beats/min on day 4 for devices coated with 5%(w/v) polymer concentration), demonstrating that the MeTro gel layer can support expansion-contraction of cells during beating. Our results indicate that elastic substrates have advantages over less elastic biomaterials in enhancing the contractile performance of CMs.
The effect of polymer concentration on the beating behavior of CMs was also investigated. CM beating was stronger inside the channels coated with 5% (w/v) MeTro but the beating rate decreased significantly with culture time. However, the beating rate was more stable over several days when higher concentrations of MeTro were used (e.g. 8% (w/v)). No significant changes were observed in the beating rate of CMs seeded inside the channels coated with various concentrations of GelMA.
In our study, the MeTro coating provided a highly elastic support for attachment, spreading, alignment, and function of CMs inside a microfluidic device to mimic the cell organization of native cardiac muscle. Our developed heart-on-a-chip platform could be used to detect the effects of physiological factors or test drugs for cardiotoxicity. For example, it could be used to investigate the dose-dependent effect of drugs on the contraction response of CMs for drug screening applications.
Our miniaturized platform offers several advantages compared to traditional in vitro methods used to engineer cardiac muscle for drug and therapeutic studies. First, our platform provides continuous medium perfusion, which allows testing the effect of continuous fluids on cardiac function. Second, our MeTro-coated device can be used to conduct multiple experiments in parallel using one chip. Third, the developed microfluidic-based platform can reduce the amount of cells and reagents and consequently decrease the costs compared to conventional techniques, especially when primary cell lines or expensive drugs are used.
In this study, we used CMs as a model cell type to engineer a heart-on-a-chip device, however, our MeTro-coated device has potential for the engineering of several microscale models of human organs such as lung, blood vessel, liver, and muscle. These organs can be later combined on a single MeTro-coated device to model a body-on-a-chip platform for drug and therapeutic studies as well as for studying the multi-tissue interactions under physiological flow conditions.
Conclusions
In this work, we have described a novel approach to coat PDMS microfluidic channels with hydrogels, introducing a cell-compatible and elastic substrate with tunable mechanical (stiffness) and physical (thickness) properties. The described experimental approach is compatible with any photocrosslinkable material, is simple and fast, and can be used to generate cell-coated polymer structures that function as support architectures for tissue constructs, e.g. in organ-on-a-chip applications. Moreover, no pre-treatment of the external PDMS structure is needed.We have shown that primary rat CMs preferentially align and proliferate on tropoelastin, compared to gelatin-based substrates. This result is in line with the observation that CMs exhibit a stronger contractile behavior on MeTro than on GelMA, indicating that further microfluidics-based research on murine cardiac tissue could benefit from utilizing tropoelastin-based hydrogel. We envision that the proposed microfluidic device structure, hydrogel coating method, and MeTro hydrogel could be combined not only for further cardiac research, but also to develop in vitro vascular structures that rely on highly elastic scaffolds. Here, we anticipate that two or more hydrogel layers could be sequentially crosslinked inside a single microfluidic channel, all of which could contain different hydrogel concentrations or types. Furthermore, it would be prudent to optimize the MeTro coating parameters (flow rate and UV intensity) to render this crosslinking method compatible with cell encapsulation. Then, we could potentially mimic the in vivo structure of a vascular channel by embedding different cell types in several MeTro layers in vitro and culturing them over an extended period of time, utilizing a simple microfluidic device.
Acknowledgements
N.A. acknowledges the support from the National Health and Medical Research Council. S.S. acknowledges funding from the US Army ORAU Postdoctoral program. A.K. acknowledges funding from the National Science Foundation CAREER Award (DMR 0847287), the Office of Naval Research Young National Investigator Award, the National Institutes of Health (HL092836, DE019024, EB012597, AR057837, DE021468, HL099073, EB008392), the Institute for Soldier Nanotechnology, the Office of Naval Research, the US Army Research Office (contract W911NF-07-D-0004), and the Presidential Early Career Award for Scientists and Engineers (PECASE). J.R. acknowledges the support from the Portuguese Foundation for Science and Technology (FCT; SFRH/BD/51679/2011). A.S.W. acknowledges funding from the Australian Research Council, Australian Defense Health Foundation and National Health & Medical Research Council. The authors thank Thomas Ferrante at the Wyss Institute at Harvard University for his technical assistance with confocal microscopy. A.S.W. is the Scientific Founder of Elastagen Pty Ltd.References
- I. Barbulovic-Nad, S. H. Au and A. R. Wheeler, Lab Chip, 2010, 10, 1536–1542 RSC.
- W. Tan and T. A. Desai, Biomaterials, 2004, 25, 1355–1364 CrossRef CAS.
- E. W. K. Young and D. J. Beebe, Chem. Soc. Rev., 2010, 39, 1036–1048 RSC.
- A. Khademhosseini, R. Langer, J. Borenstein and J. Vacanti, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 2480–2487 CrossRef CAS.
- C. A. Bichsel, S. Gobaa, S. Kobel, C. Secondini, G. N. Thalmann, M. G. Cecchini and M. P. Lutolf, Lab Chip, 2012, 12, 2313–2316 RSC.
- C. D. Chin, T. Laksanasopin, Y. K. Cheung, D. Steinmiller, V. Linder, H. Parsa, J. Wang, H. Moore, R. Rouse, G. Umviligihozo, E. Karita, L. Mwambarangwe, S. L. Braunstein, J. V. D. Wijgert, R. Sahabo, J. E. Justman, W. El-Sadr and S. K. Sia, Nat. Med., 2011, 17, 1015–1019 CrossRef CAS.
- S. Schumacher, J. Nestler, T. Otto, M. Wegener, E. Ehrentreich-Forster, D. Michel, K. Wunderlich, S. Palzer, K. Sohn, A. Weber, M. Burgard, A. Grzesiak, A. Teichert, A. Brandenburg, B. Koger, J. Albers, E. Nebling and F. F. Bier, Lab Chip, 2012, 12(3), 464–473 RSC.
- P. S. Dittrich and A. Manz, Nat. Rev. Drug Discovery, 2006, 5, 210–218 CrossRef CAS.
- J. Pihl, J. Sinclair, E. Sahlin, M. Karlsson, F. Petterson, J. Olofsson and O. Orwar, Anal. Chem., 2005, 77, 3897–3903 CrossRef CAS.
- Y. Wen and S.-T. Yang, Expert Opin. Drug Discovery, 2008, 3, 1237–1253 CrossRef CAS.
- A. M. Ghaemmaghami, M. J. Hancock, H. Harrington, H. Kaji and A. Khademhosseini, Drug Discovery Today, 2012, 17, 173–181 CrossRef CAS.
- D. Huh, G. A. Hamilton and D. E. Ingber, Trends Cell Biol., 2011, 21, 745–754 CrossRef CAS.
- D. B. Weibel and G. M. Whitesides, Curr. Opin. Chem. Biol., 2006, 10, 584–591 CrossRef CAS.
- B. J. Battersby and M. Trau, Trends Biotechnol., 2002, 20, 167–173 CrossRef CAS.
- C. Yi, C.-W. Li, S. Ji and M. Yang, Anal. Chim. Acta, 2006, 560, 1–23 CrossRef CAS.
- F. Teruo, Microelectron. Eng., 2002, 61–62, 907–914 Search PubMed.
- S. Selimovic, F. Gobeaux and S. Fraden, Lab Chip, 2010, 10, 1696–1699 RSC.
- M. B. Romanowsky, M. Heymann, A. R. Abate, A. T. Krummel, S. Fraden and D. A. Weitz, Lab Chip, 2010, 10, 1521–1524 RSC.
- B. V. Slaughter, S. S. Khurshid, O. Z. Fisher, A. Khademhosseini and N. A. Peppas, Adv. Mater., 2009, 21, 3307–3329 CrossRef CAS.
- H. Aubin, J. W. Nichol, C. B. Hutson, H. Bae, A. L. Sieminski, D. M. Cropek, P. Akhyari and A. Khademhosseini, Biomaterials, 2010, 31, 6941–6951 CrossRef CAS.
- V. A. Liu and S. N. Bhatia, Biomed. Microdevices, 2002, 4, 257–266 CrossRef CAS.
- A. Townsend-Nicholson and S. N. Jayasinghe, Biomacromolecules, 2006, 7, 3364–3369 CrossRef CAS.
- W. Lee, J. C. Debasitis, V. K. Lee, J.-H. Lee, K. Fischer, K. Edminster, J.-K. Park and S.-S. Yoo, Biomaterials, 2009, 30, 1587–1595 CrossRef CAS.
- D. Hellio and M. Djabourov, Macromol. Symp., 2006, 241, 23–27 CrossRef CAS.
- J. W. Nichol, S. Koshy, H. Bae, C. M. Hwang, S. Yamanlar and A. Khademhosseini, Biomaterials, 2010, 31, 5536–5544 CrossRef CAS.
- A. Khademhosseini, G. Eng, J. Yeh, J. Fukuda, J. Blumling, 3rd, R. Langer and J. A. Burdick, J. Biomed. Mater. Res. A, 2006, 79, 522–532 CrossRef.
- M. Collins and C. Birkinshaw, J. Mater. Sci.: Mater. Med., 2008, 19, 3335–3343 CrossRef CAS.
- A. Patel, M. Sandig and K. Mequanint, Cardiovasc. Res., 2006, 71, 40–49 CrossRef CAS.
- S. M. Mithieux, J. E. J. Rasko and A. S. Weiss, Biomaterials, 2004, 25, 4921–4927 CrossRef CAS.
- J. Rnjak, W. Daamen, M. Pierna, J. C. Rodríguez-Cabello and A. S. Weiss, in Comprehensive Biomaterials, ed. P. Ducheyne, K. E. Healy, D. W. Hutmacher, D. Grainger and C. J. Kirkpatrick, Elsevier, 2011, vol. 2, pp. 329–346 Search PubMed.
- N. Annabi, S. M. Mithieux, P. Zorlutuna, G. Camci-Unal, A. S. Weiss and A. Khademhosseini, Biomaterials, 2013, 34, 5496–5505 CrossRef CAS.
- W. J. Wu, B. Vrhovski and A. S. Weiss, J. Biol. Chem., 1999, 274, 21719–21724 CrossRef CAS.
- F. Xu, T. Beyazoglu, E. Hefner, U. A. Gurkan and U. Demirci, Tissue Eng., Part C, 2011, 17, 641–649 CrossRef.
- H. A. Stone, A. D. Stroock and A. Ajdari, Annu. Rev. Fluid Mech., 2004, 36, 381–411 CrossRef.
- T. Squires and S. Quake, Rev. Mod. Phys., 2005, 77, 977–1026 CrossRef CAS.
- N. Annabi, K. Tsang, S. M. Mithieux, M. Nikkhah, A. Ameri, A. Khademhosseini and A. S. Weiss, Adv. Funct. Mater. DOI:10.1002/adfm.201300570.
- D. V. Bax, U. R. Rodgers, M. M. Bilek and A. S. Weiss, J. Biol. Chem., 2009, 284, 28616–28623 CrossRef CAS.
- A. W. Feinberg, A. Feigel, S. S. Shevkoplyas, S. Sheehy, G. M. Whitesides and K. K. Parker, Science, 2007, 317, 1366–1370 CrossRef CAS.
- C. A. Bashur, L. Venkataraman and A. Ramamurthi, Tissue Eng., Part B: Rev., 2012, 18, 203–217 CrossRef CAS.
- A. C. Mattiello-Sverzut, L. Chimelli, S. Teixeira, M. Pierre and L. Oliveira, Braz. J. Med. Biol. Res., 2005, 38, 303–307 CrossRef CAS.
- P. Panisello, J. R. Torrella, T. Pagés and G. Viscor, High Alt. Med. Biol., 2007, 8, 322–330 CrossRef.
- G. C. Engelmayr, Jr., M. Cheng, C. J. Bettinger, J. T. Borenstein, R. Langer and L. E. Freed, Nat. Mater., 2008, 7, 1003–1010 CrossRef.
- K. Baar, R. Birla, M. O. Boluyt, G. H. Borschel, E. M. Arruda and R. G. Dennis, FASEB J., 2005, 19, 275–277 CAS.
- S. M. LaNasa and S. J. Bryant, Acta Biomater., 2009, 5, 2929–2938 CrossRef CAS.
- S. Y. Boateng, S. S. Lateef, W. Mosley, T. J. Hartman, L. Hanley and B. Russell, Am. J. Physiol. Cell Physiol., 2005, 288, C30–38 CAS.
- A. Khademhosseini, G. Eng, J. Yeh, P. A. Kucharczyk, R. Langer, G. Vunjak-Novakovic and M. Radisic, Biomed. Microdevices, 2007, 9, 149–157 CrossRef.
- M. Radisic, L. Yang, J. Boublik, R. J. Cohen, R. Langer, L. E. Freed and G. Vunjak-Novakovic, Am. J. Physiol.: Heart Circ. Physiol., 2004, 286, 507H–516 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3lc50252j |
| ‡ These authors contributed equally to this article. |
| This journal is © The Royal Society of Chemistry 2013 |
