An enclosed in-gel PCR amplification cassette with multi-target, multi-sample detection for platform molecular diagnostics†
Dammika P.
Manage
a,
Jana
Lauzon
a,
Alexey
Atrazev
a,
Ravi
Chavali
ab,
Roshini A.
Samuel
c,
Brandon
Chan
a,
Y. C.
Morrissey
a,
Walter
Gordy
d,
Ann L.
Edwards
a,
Kyle
Larison
a,
Stephanie K.
Yanow
ce,
Jason P.
Acker
b,
George
Zahariadis
cbf and
Linda M.
Pilarski
*ab
aDepartment of Oncology, University of Alberta, 11560 University Avenue, Edmonton, AB T6G 1Z2, Canada. E-mail: lpilarsk@ualberta.ca; Fax: +1 780 432 8425; Tel: +1 780 432 8925
bDepartment of Laboratory Medicine and Pathology, University of Alberta, 8440 - 112 Street, Edmonton, AB T6G 2B7, Canada
cProvincial Laboratory for Public Health, 8440 - 112 St, Edmonton, AB T6G 2J2, Canada
dAquila Diagnostic Systems Inc., Edmonton Alberta, Canada
eSchool of Public Health, University of Alberta, 8303 - 112 St., Edmonton, AB T6G 2T4, Canada
fVictoria Hospital, London Health Sciences Centre and Department of Pathology, Western University Schulich School of Medicine, 800 Commissioners Rd E, London, ON N6A 5W9, Canada
First published on 7th March 2013
Abstract
This work describes a self-contained, simple, disposable, and inexpensive gel capillary cassette for DNA amplification in near point of care settings. The cassette avoids the need for pumps or valves during raw sample delivery or polymerase chain reaction (PCR) amplification steps. The cassette contains capillary reaction units that can be stored at room temperature for up to 3 months. The current cassette configuration format simultaneously tests up to 16 patients for two or more targets, accommodates different sample types on the same cassette, has integrated positive and negative controls and allows flexibility for multiple geometries. PCR reagents in the cassette are desiccated to allow storage at room temperature with rehydration by raw sample at the time of testing. The sample is introduced to the cassette via a transfer pipette simply by capillary force. DNA amplification was carried out in a portable prototype instrument for PCR thermal cycling with fluorescence detection of amplified products by melt curve analysis (MCA). To demonstrate performance, raw genital swabs and urine were introduced to the same cassette to simultaneously detect four sexually transmitted infections. Herpes Simplex Viruses (HSV-1 and HSV-2) were detected from raw genital swabs. Ureaplasma urealyticum (UU) and Mycoplasma homonis (MH) were detected from raw urine. Results for multiple patients were obtained in as little as 50 min. This platform allows multiparameter clinical testing with a pre-assembled cassette that requires only the introduction of raw sample. Modification of the prototype device to accommodate larger cassettes will ultimately provide high throughput simultaneous testing of even larger numbers of samples for many different targets, as is required for some clinical applications. Combinations of wax and/or polymer cassettes holding capillary reaction units are feasible. The components of the cassette are suited to mass production and robotic assembly to produce a readily manufactured disposable reaction cassette that can be configured for disease-specific testing panels. Rapid testing with a disposable reaction cassette on an inexpensive instrument will enable on the spot evaluation of patients in the clinic for faster medical decision-making and more informed therapeutic choices.
1 Introduction
Recent advances in developing lab on a chip (LOC) devices aim to move central clinical laboratory testing into individual clinics or to the hospital bedside for near point of care (near-POC) diagnostics. These advances will play a key role in rapid detection of diseases such as sexually transmitted infections (STIs) thereby facilitating early intervention. Annually, about 448 million new cases of STIs are diagnosed globally.1 Even developed countries with high end healthcare infrastructure and public health resources are not immune to alarming increases in incidence of STIs.2Herpes Simplex Viruses 1 and 2 (HSV-1 and HSV-2) are two of the most predominant STIs; they cause chronic infections with sporadic reactivation, and maternal transmission can pose a severe threat to newborns.3–5 Mycoplasma and ureaplasma infections such as Mycoplasma homonis (MH) and Ureaplasma urealyticum (UU) are emerging STIs that cause symptomatic or asymptomatic infections. These pathogens affect reproductive health, are linked to preterm labour and can have severe outcomes if there is maternal transmission to a newborn.6–11 In developed countries, confirmation of an STI occurs mainly in central laboratories, either by culture methods or by molecular testing which requires highly trained personnel and complex instruments. This process can take several days to weeks for a diagnosis with consequent delays in treatment and potential loss of patient follow-up. Near-POC testing performed while a patient is assessed in the clinic will allow accurate identification of the causative STI. This is invaluable for offering immediate and appropriate treatment to the patient (and their partners) and could have a significant public health impact by reducing transmission and ultimately prevalence of these infections.
Recently, several on-chip PCR based devices have been reported for detection of infectious agents.12–14 These chips detect only one agent in a given sample, require sample preparation steps and lack integrated positive and negative controls. Pan et al. designed a glass chip with four-chambers and microfluidic multi-channels for detecting amplicons by capillary electrophoresis.15 All of these chips require fabricated fluidic channels, a means to pump reagents through channel networks and manual loading of PCR reagents immediately prior to amplification. Kim et al. demonstrated use of Illustra PuRe Taq Ready-To-Go PCR beads that allow storage at ambient temperatures with dried reagents encapsulated inside a chip-based PCR chamber by a paraffin film; this system detects one agent in one sample and lacks positive and negative controls.16
Previously, we reported in-gel PCR in 670–860 nL of cylindrical or conical hydrogels (gel posts).17,18 HSV was detected with raw genital swabs in an open array of freshly made gel posts.18 We performed a study of 45 clinical samples that were blinded to the operator and obtained a concordance of 91% with the gold standard diagnostic and confirmed the reliability of gel-based DNA amplification in individual reaction units configured as an array. However, gel posts cannot be manufactured and must be enclosed if they are to be used in the clinic. Here we developed and verified a gel-based cassette that is amenable to manufacture with robotic assembly, is stable for “on the shelf” storage and provides a multiparameter system for simultaneous testing of multiple samples and targets on the same cassette. The cassette is pre-assembled with all reaction components except the sample that contains the template. Our pre-assembled cassette requires only that a clinician, nurse, or technician flow raw samples into the cassette (the disposable unit), insert into the instrumentation (currently in prototype form), and press “go”. The testing involves DNA amplification (PCR) followed by melt curve analysis (MCA), which collectively take less than an hour. Data analysis is currently manual, taking 5–7 min with anticipated software improvements.
The ready-made cassette contains short glass capillaries holding desiccated acrylamide gels with all the reagents required for the PCR reaction except the DNA template. These are arranged in wax trenches on a metal pan. Positive and negative controls are included in the cassette. All cassette components are commercially available and low-cost. For the work presented here, the capillaries contain reagents for detecting four sexually transmitted infections. To enable on the shelf storage of cassettes, the gel mixture inside capillaries is desiccated prior to cassette assembly and the final cassette is vacuum-packed in plastic. When testing occurs, the cassette is removed from the packaging and raw sample containing the templates is introduced to the desiccated gel mixture by capillary action to rehydrate the capillaries.
Many POC or near-POC devices face the challenge of sealing the amplification chamber during the PCR to prevent sample/reagent evaporation. Applying pressure to seal the perimeter of the chamber is widely used where a lid makes a tight seal or a pressured finger is used to pinch the channels. Another way is to add mineral oil to immerse the reagents as described for the gel post system17–19 and also by Reboud et al.20 The use of wax to enclose capillaries provides a vapour barrier that avoids the need for complex pressure-based sealing techniques and bypasses the need for fluid agents in a packaged reaction cassette. After PCR is complete, the wax solidifies again to enclose the used capillaries for safe disposal. The hydrogel-filled capillaries and cassette are currently made on a bench top without any need for complex fabrication. The cassette design is very flexible and can accommodate multiple capillary geometries for testing of multiple samples and/or sample types. The configuration chosen for this current proof of principle accepts up to 16 patient samples and/or sample types and multiple targets with integrated negative and positive controls.
2 Materials and methods
2.1 Samples
Genital swab samples (HSV-1 positive, HSV-2 positive, and negative controls-based on testing using culture methods) were obtained from the Alberta Provincial Laboratory for Public Health. Genital swabs from patients were placed in universal transport media (UTM; Copan Diagnostics Inc., Murrieta, CA, USA) at the clinic and transported to the laboratory where they were frozen at −20 °C until use. For all experiments, unprocessed samples in UTM were used directly without DNA purification. Thirty anonymized urine samples were obtained from the Edmonton Sexually Transmitted Disease Clinic from all patients visiting on a specific day. Unprocessed urine samples were also used without DNA purification. Collection of anonymized clinical samples was approved by the Health Research Ethics Board of the University of Alberta.2.2 Reagents
Separate reaction mixes were prepared with (positive controls) or without template for HSV-1, HSV-2, MH, and UU and used to fill capillaries. The primer sets for detecting HSV-1, HSV-2, UU and MH are shown in Table 1 in the ESI.† Each 100 μL reaction mix consisted of 20 μL of 5× PCR buffer (333 mM tris-sulfate, pH 8.6, 83 mM (NH4)2SO4 (Sigma, St. Louis, MO.); and 40% sucrose (Sigma)), 30 μL of 40% sugar, 4 μL of 50 mM MgCl2 (Fluka, Buchs), 2 μL of 10 mM [dNTP] (Sigma), 2 μL of 1% BSA (Sigma), 4 μL of 10 μM primer solution (Integrated DNA technologies, San Diego, CA) for each of the two primers, 10 μL of 10× LC Green Plus (Idaho Technology Inc., Salt Lake City, Utah) and 4 μL of Taq polymerase (20 units/μL), 10 μL of a 40% acrylamide (Sigma) + 4% bis-acrylamide aqueous solution (N,N-methylene bisacrylamide, BioRad, Hercules, CA), 2 μL of 3% azobis (Wako, Richmond), 1 μL of 10% TEMED (N,N,N′,N′-tetramethylethylenediamine, Sigma) and water. For the positive controls, 4 μL of sample was added, replacing water. The mixes were vortexed, centrifuged, and loaded into the capillaries as described below.2.3 Overview of the entire system
The complete concept of the system including the prototype instrument presented in this paper is summarized in Fig. 1. Each step in preparation of the cassette as well as performing the PCR and MCA are described in detail below. Preparation of reaction units is given in Fig. 2(a). Details for the instrument are provided in ESI, Appendix 2.†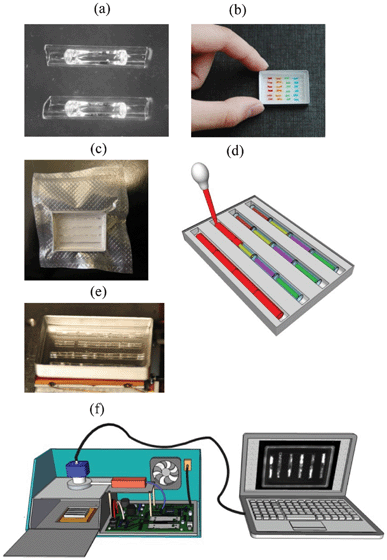 | ||
| Fig. 1 Overview of the presented system: (a) dried gel inside capillary reaction units; (b) a cassette with capillaries arranged in 6 trenches. Capillaries are colored to represent different primer sets; (c) a vacuum packaged cassette, (d) sample delivery with a transfer pipette to each trench of the cassette from one end of the trench. The sample flows smoothly through the entire trench and hydrates gel in the capillaries; (e) a cassette with a 4 trench capillary arrangement in melted wax during thermal cycling; (f) a diagram of a second generation PCR/MCA instrument (GelCycler) (see ESI†). This instrument has capabilities similar to the previously described first generation instrumentation17,18 but with much improved temperature regulation and detection of emitted light. | ||
2.4 Preparation of glass capillaries
Glass hemotocrit tubes (Plain, Blue Tip-Fisher Scientific, Fair Lawn, NJ.) were cut to 6 or 7 mm in length. The inner diameter of the capillary is 1.1 mm while the outer diameter is 1.5 mm.The steps for the preparation of gel capillaries for PCR are shown in Fig. 2a. Each set of capillaries was filled by dipping one end into the appropriate reaction mix and placed on a cover glass or a petri dish. Capillaries that served as positive controls were filled with the reaction mix containing the specified primer set and a known positive sample while all the others were filled with reaction mix and the specified primer set but no template. Capillaries were then exposed to 360 nm UV light (∼1 mW cm−2 on the capillaries) for 30 min in order to photo-polymerize the gel/reaction mix as 4% acrylamide gel capillaries. They were then desiccated as described below.
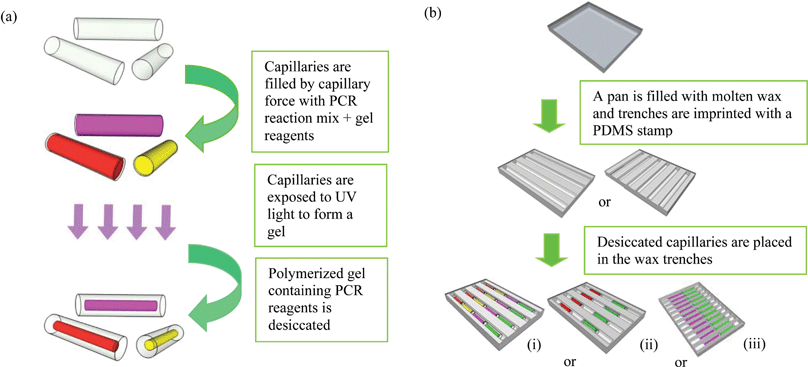 | ||
| Fig. 2 Procedure for making cassettes with capillaries: (a) steps for making capillaries: capillaries are cut to 6 or 7 mm in length, filled with PCR/gel reaction mix, exposed to the UV light for photo-polymerization, and desiccated to create a dried gel “noodle” inside the capillaries. (b) Steps for making cassettes: in an aluminium pan, wax is imprinted to create trenches with different arrangements for placing capillaries; (i)–(iii): examples of formats for assembly of capillaries with different primer sets and positive controls. Each color represents a capillary with a different set of primers. Each capillary holds from 6–7 microliters of mixture. | ||
2.5 Desiccation of the gel capillaries
The polymerised capillaries were placed in a vacuum oven (Fisher Scientific) to desiccate the gel. After desiccation, capillaries hold dried gel in the shape of a “noodle” as shown in Fig. 1(a). For delivering the sample to the capillary, the noodle shape of the dried gel is vital as the space created between the gel noodle and the glass capillary walls creates a path for the sample to flow by capillary force and thereby rehydrate the gel.2.6 Enclosure and storage of capillaries inside a cassette
A polymer cassette with trenches can also be used to hold capillary reaction units. As proof of principle, 9 parallel trenches 1.6 mm in width with rounded bottoms were milled in a sheet of thermally conductive polymer (Cool Polymers Inc., North Kingstown, RI, USA) with dimensions of 29 mm × 25 mm × 3 mm. The thickness of the polymer at the bottom of the trench is 0.4 mm. Each trench was filled with 80 μL of wax prior to arranging the capillaries.
In order to study the stability of the reagents at room temperature for long term storage, separate sets of stored cassettes were then tested after 1 week, 1.5 months, and 3 months.
2.7 DNA amplification in gel capillary cassettes
The 30 anonymized urine samples were also screened by conventional PCR to identify those with UU and MH infections. The reaction mix is as above except that 2.5 μL of urine was added to the 25 μL reaction mix. Thermocycling conditions were similar to conventional PCR for HSVs.
After the sample was introduced into the cassette, the rehydrated cassette was then placed on the peltier element of the GelCycler for thermal cycling (Fig. 1e). After a pre-denaturation step of 3 min at 94 °C, 35 cycles of DNA amplification were carried out at 94 °C for 5 s, 60 °C for 10 s, and 72 °C for 10 s, followed by final extension step of 120 s at 72 °C. During the PCR, CCD images were taken at 5 s into each extension step. MCA was performed from 70–95 °C and CCD images were taken at every 0.2 °C.
For testing on cassettes, the CCD images acquired during PCR and MCA were analyzed to visualize the amplification status and to detect the products in each capillary. Images taken at the extension step of each PCR cycle (35 or 40) were analyzed with ImageJ software (National Institutes of Health, U.S.) using the MicroArray Rectangular Plug-in (Dr Robert Dougherty, OptiNav Inc., Redmond, WA) to plot the cycle number vs. the fluorescence intensity in each capillary. Using the software, rectangles were placed around the image of each capillary in the first image, followed by automated analysis of the sequential image series to measure the fluorescence intensity of each capillary during PCR and MCA. The image recognition software acquires this data without human intervention.
Melting curves were created from CCD images taken at 0.2 °C degree interval during the heating of the cassette from 70 °C to 95 °C and were analyzed to measure the melting temperature for amplicons in each capillary, as previously described.17,18 A plot of the negative derivative of this fluorescence with respect to the temperature determines the melting temperature (Tm) of the PCR products.
3 Results and discussion
Rationale: We have previously described hydrogel-based PCR systems incorporating multiparameter PCR on individual reaction units termed gel posts,17,18 using mineral oil as the vapour barrier. While this proved to be a reliable strategy, the gel post chips are not readily manufacturable and oil creates problems for handling of a packaged cassette. To create a system that could be easily manufactured, we redesigned the platform as a cassette comprised of hydrogel in capillaries, using “off the shelf” materials, each capillary serving as an individual reaction unit with a specified primer set, as described below. A chosen complement of capillary reaction units with the required primer sets can be assembled in wax trenches. The wax then immobilizes capillaries in the trench, thereby enclosing them in a cassette, a process that is amenable to eventual robotic assembly. When PCR cycling begins, the wax melts and creates a vapour barrier. When PCR is complete, the wax solidifies and allows safe disposal of used cassettes. These assembled capillaries each holding a desiccated hydrogel reaction mixture create a reaction cassette that can be stored at ambient temperature and later rehydrated by raw sample. Desiccation enables rapid sample delivery creating a channel within each capillary that allows raw sample to enter via capillary action. A given cassette tests multiple patient samples and/or multiple sample types for multiple targets with no cross-contamination. Each cassette includes quality control verification through integrated positive and negative controls for each target. The desiccated capillary reaction units are stable over time, thus enabling “on the shelf” storage of assembled cassettes.3.1 Cassette PCR employs wax as a vapour barrier
In our previous work, we amplified DNA in gel posts that were immersed in oil.17–19 A comparison of oil and wax as vapour barriers for gel-filled capillaries is shown in ESI, Appendix 3,† where HSV-1 or HSV-2 in raw genital swabs were amplified on cassettes in wax or covered in mineral oil. Template DNA was polymerized into the gel along with the other reagents, using freshly filled and polymerized gel in capillaries. Negative controls lacked DNA template. Real-time PCR data and MCA data shown in ESI, Appendix 3,† using oil and wax as vapour barriers, are very similar indicating that both media provide a suitable vapour barrier to keep the gels hydrated during thermal cycling and MCA.Capillaries are readily filled with gel mixture and are flexible in terms of using different primer sets in assembled cassettes, with no cross-contamination as shown above and in later sections. However, for use in a clinical setting, pre-made cassettes should have a prolonged shelf life, with unprocessed samples introduced at the time of testing. The use of freshly polymerized gels is incompatible with these requirements as sample cannot be added to a capillary after polymerization. We therefore developed a desiccation strategy that allows sample to be introduced during a rehydration step. After filling capillaries with gel reaction mixture, the gel inside the capillaries was desiccated. Desiccated gel accounts for about 15–18% of the whole capillary volume and holds a shape of a noodle inside the gel, which is essential for delivery of the sample to the capillary which rehydrates the gel at the time of testing.
3.2 PCR Cassettes can be stored for a prolonged period
A set of experiments was designed to confirm that PCR reagents in desiccated capillary reaction units could still support PCR and were stable at room temperature for at least 3 months, shown in Fig. 3. PCR amplification and MCA were performed after desiccation and storage for 1 week, 1.5 months, and 3 months. Cassettes contained desiccated capillary units for detecting HSV-1 and HSV-2 as well as negative and positive controls.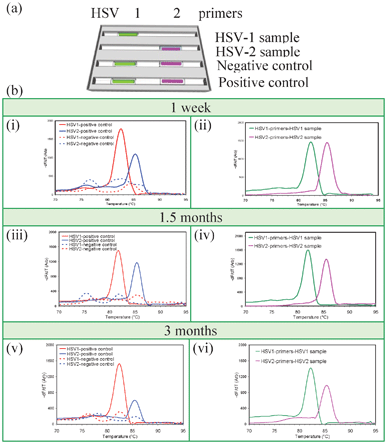 | ||
| Fig. 3 Long-term storage of vacuum-sealed desiccated gel cassettes. (a) Capillary arrangement. Capillaries in the first two trenches test samples for HSV-1 and HSV-2 while the third and fourth trenches contain negative and positive control capillaries. The first and second trenches were rehydrated with HSV-1 and HSV-2 samples, respectively, while the third and fourth trenches were rehydrated with water. (b) MCA data after storage for one week (i, ii), 1.5 months (iii, iv), and 3 months (v, vi). Real time quantitative PCR Cp values after one week, 1.5 month, and 3 months are 23.2, 23.5. and 21.4 for HSV-1 sample, 27.2, 26.5, and 26.0 for HSV-2 sample, 21.4, 20.3, and 19.8 for HSV-1 positive control, and 27.6, 27.3, and 27.2 for HSV-2 positive control, respectively, showing that stored reaction units were comparable for all time points, indicating equivalent amplification capabilities over the time. | ||
MCA data from Fig. 3 shows that the reagents within the cassette maintain activity for at least three months of storage at room temperature. Cp values were equivalent to controls for all time points (Fig. 3), indicating comparable amplification. This suggests that cassettes holding gel-filled capillaries are feasible for detecting pathogens in resource-limited areas of the world where refrigeration and power are erratic or unavailable.
For all subsequent experiments, cassettes were pre-made and stored in vacuum-sealed bags at room temperature for at least several days before use. Air-tight sealing during storage was essential to prevent any further loss of moisture that can compromise enzyme activity.
3.3 Cassette PCR enables a high sensitivity of HSV detection in genital swabs
In order to test the sensitivity of the cassette system, PCR was performed with a dilution series of genital swabs positive for HSV-1 or HSV-2. Serial dilutions of the swab samples were made at dilution factors of 10−1, 10−2, 10−3, and 10−4 which were then used to hydrate the gel capillaries. The cassette was arranged as shown in Fig. 4a(i) and the fluorescence image of the cassette at the 35th PCR cycle is shown in Fig. 4a(ii)). The PCR data is shown in Fig. 4a(iii) and 4a(iv) and MCA data is shown in Fig. 4a(v) and 4a(vi) for HSV-1 and HSV-2, respectively.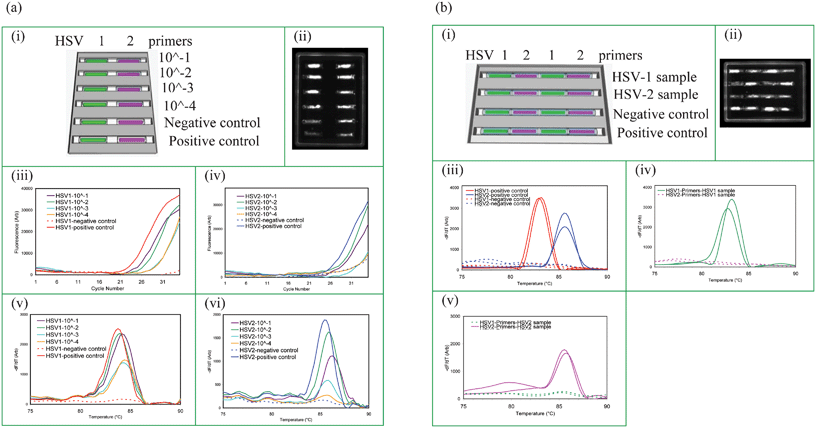 | ||
| Fig. 4 Test sensitivity and absence of cross-contamination. (a) Test sensitivity for detecting STIs in raw HSV positive genital swab samples (i) capillary layout. The cassette contains positive and negative controls. (ii) CCD image at 35th cycle of PCR, real-time PCR amplification curves: (iii) HSV-1 and (iv) HSV-2, MCA profiles: (v) HSV-1 and (vi) HSV-2 for different dilutions of the swab samples. (b) Absence of cross-contamination between capillaries with different primers: (i) capillary layout and the sample delivery information for a cassette with 4 trenches where each trench contains HSV-1 and HSV-2 test capillaries alternately placed. HSV-1 and HSV-2 samples were tested in trenches 1 and 2, respectively, while trench 3 is a negative control and trench 4 is the positive control. (ii) CCD image at 35th cycle of PCR, MCA curves: (iii) positive and negative controls-trenches 3 and 4, (iv) HSV-1 sample delivery to trench 1, and (v) HSV-2 sample delivery to trench 2. PCR products were also sequenced to confirm their identities. Real-time PCR data for each capillary is given in ESI, Appendix 4.† | ||
The absolute number of HSV copies from a genital swab is not reported routinely, and is generally not clinically meaningful. Fig. 4a shows that HSV-1 is readily detectable in as little as a 1/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dilution of a genital swab and HSV-2 is weakly detectable in a 1/10
000 dilution of a genital swab and HSV-2 is weakly detectable in a 1/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dilution, an improvement over our previous system18 where 1/10
000 dilution, an improvement over our previous system18 where 1/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dilution of a genital swab was shown to equate to detection of ∼6 copies of HSV DNA, based on DNA standards with known HSV copy numbers. This suggests that very low HSV copy numbers from clinically relevant genital herpes infections are likely to be detectable using this system.
000 dilution of a genital swab was shown to equate to detection of ∼6 copies of HSV DNA, based on DNA standards with known HSV copy numbers. This suggests that very low HSV copy numbers from clinically relevant genital herpes infections are likely to be detectable using this system.
3.4 Absence of cross contamination in cassette PCR
Capillaries with desiccated gel noodles each containing a primer set for a different pathogen can be arranged in wax trenches such that the sample can be delivered from one end to the all of the capillaries in a given trench as shown in Fig. 1(c). These cassettes can be manufactured to perform multiparameter PCR with different testing panels for simultaneous detection of multiple pathogens. However, it was critical to confirm that the different primers or amplicons from adjacent capillaries do not cross-contaminate each other.Cassettes having 4 trenches with four 6 mm capillaries per trench were arranged with alternate HSV-1 and HSV-2 capillaries physically adjacent to each other within the trench along which sample flows. This is shown in Fig. 4b(i) where the first two trenches receive samples, and trenches 3 and 4 receive water for the negative and positive controls. Samples were from patients with known HSV status.
Experiments to evaluate cross contamination are shown in Fig. 4b. Fig. 4b(iii–v) shows that each capillary produces only the correct product; the capillary with HSV-1 primers only amplifies HSV-1 positive samples but not HSV-2 positive samples and vice versa, regardless of the reaction specificity of neighboring capillaries in the trench. This can be seen in the CCD image shown in Fig. 4b(ii). These results show that the primers or amplicons in adjacent capillaries do not travel or diffuse outside their own boundaries and hence no cross contamination is detectable during the sample delivery or during the rehydration process. We observed that as PCR begins, the wax melts and the openings of each capillary are sealed instantly thereby preventing any communication between the capillaries during the PCR and MCA.
These results also confirm primer specificity and highlight the importance of MCA for the correct interpretation of fluorescence data. The amplified products from the gel were extracted from capillaries and sequenced to confirm their identity. Any primer-dimers or incorrect amplification will contribute to overall fluorescence and hence influence the amplification curve, giving an invalid Cp value. For this reason, MCA is essential to confirm product identity by observing a defined melt peak at the correct Tm for the expected amplicon.
3.5 Cassette PCR enables multi-target screening of samples from multiple patients
A cassette with capillaries arranged in an aluminium pan to simultaneously detect 2 STIs in multiple patients (HSV-1 and HSV-2) is shown in Fig. 5a. The capillaries are 6 mm in length. The CCD image at 30th cycle of PCR is shown in Fig. 5a(i). The MCA data for negative and positive controls are shown in Fig. 5a(iii) while the data for 8 different samples are shown in Fig. 5a(iv–xi), with one sample applied per trench. This experiment further confirms that the capillaries do not communicate or contaminate each other during sample delivery, the rehydration process or during the PCR and MCA.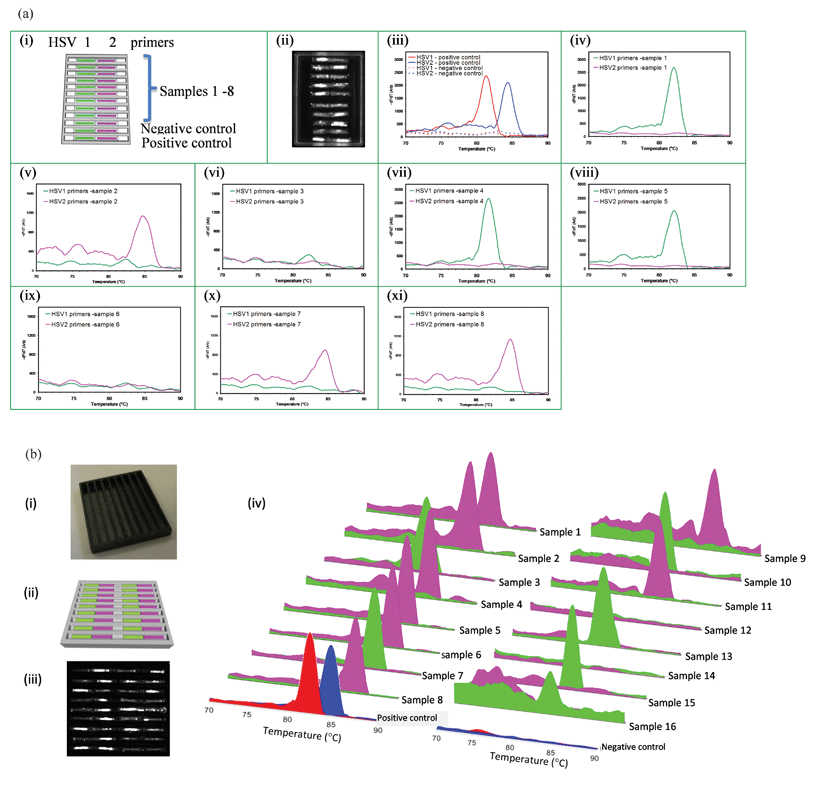 | ||
| Fig. 5 Testing multiple samples in wax or polymer cassettes. (a) Simultaneous detection of STIs in samples from 8 different patient samples on the same wax cassette: (i) capillary layout. The first 8 trenches detect 8 samples while 9th trench is a negative control and the 10th trench is the positive control. (ii) CCD image at 30th cycle of PCR, MCA profiles: (iii) positive and negative controls, (iv) sample 1 (HSV-1), (v) sample 2 (HSV-2), (vi) sample 3 (Negative), and (vii) sample 4 (HSV-1), (viii) sample 5 (HSV-1), (ix) sample 6 (Negative), (x) sample 7 (HSV-2), and (xi) sample 8 ((HSV-2). (b) Simultaneous testing for STIs of 16 patient samples using a polymer cassette: (i) photograph of the polymer pan with trenches, (ii) capillary layout. The first 8 trenches accept 16 different samples while the 9th trench contains positive and negative controls, Green and magenta represent the capillaries containing HSV-1 and HSV-2 primers respectively. (iii) CCD image at 35th cycle of PCR, and (iv) MCA curves for all 16 samples and positive and negative controls (HSV-1 and HSV-2 are red and blue respectively). All 16 samples were accurately identified including two negative samples (#12, #14). | ||
A cassette with a black polymer pan is capable of testing 16 HSV raw genital swab samples is shown in Fig. 5b(i) and 5b(ii) respectively. The use of black polymer prevents fluorescence bleed that can otherwise occur when capillaries are too closely proximate. During thermal cycling the wax in the polymer trenches melts and capillaries sink to the bottom of the trench, providing for improved thermal contact with the pan. MCA results in Fig. 5b(iv) show results from a large number of samples in adjacent trenches, with two different samples per trench simultaneously tested for two STIs, in the same cassette. There is no detectable cross-contamination despite the close proximity of samples and reaction units.
3.6 Cassette PCR allows simultaneous testing on the same cassette of genital swabs and urine, for multiple targets
HSV-1, HSV-2, UU and MH targets were amplified on the same cassette with genital swabs tested for HSVs and urine samples tested for mycoplasma targets (UU and MH). The capillary arrangement for a four target STI panel is shown in Fig. 6(i). These data show the capability of the cassette for detecting multiple infections in multiple sample types from one or more patients. It further confirms that amplification occurs independently in each capillary with no cross contamination along the trench.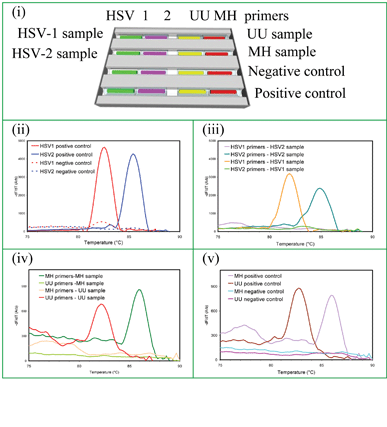 | ||
| Fig. 6 Simultaneous detection of 4 STIs in two different types of clinical sample (genital swabs and urine): (i) capillary arrangement for and sample types delivered to a wax cassette to detect HSV-1 and HSV-2 from genital swabs on the same cassette with UU and MH from urine. MCA data: (ii) positive and negative controls for HSV-1 and HSV-2, (iii) HSV-1 and HSV-2 sample detection, (iv) positive and negative controls for UU and MH, and (v) UU and MH sample detection. The melt temperatures of UU and MH are 82 °C and 86 °C, respectively. | ||
4 Conclusions
We have demonstrated a ready to use, disposable, inexpensive near-POC gel capillary cassette made from “off the shelf” materials. The cassette has integrated negative and positive controls and can perform molecular analysis in under an hour with raw genital swab and urine samples. At the time of testing, samples are introduced to this pre-assembled and packaged cassette. Individual gel capillaries in the cassette have different primers to independently detect multiple targets (multiparameter). The cassettes can test multiple patients with multiple sample types for multiple targets in ∼50 min. The reaction units in the cassette are short glass capillaries with desiccated PCR reagents in hydrogel. Capillaries loaded with different primers are arranged in a wax-filled pan to create a cassette for defined testing panels. This cassette can be stored at room temperature for up to 3 months. The sample is delivered to the cassette with a transfer pipette to rehydrate the gels, with no need for pumps or valves at any stage of PCR or MCA. The wax trenches for capillary assembly in the cassette serve as a vapour barrier once PCR cycling begins. Melted wax also forms barriers between adjacent capillaries preventing cross-contamination among primers or amplicons. As a proof of principle, we show the use of raw genital swab and raw urine samples to detect 4 different STIs from two different patients on the same cassette. Once results are obtained and cycling ends, the wax solidifies for easy biosafe disposal.The cassette design is extremely flexible and can accommodate a wide variety of configurations. The cost is less than 12 cents per 6 mm capillary, making the cost for a typical cassette less than two dollars, including the pan and the wax. Robotic loading of reaction mixture into capillaries and robotic placement of desiccated reaction units in trenches seems feasible. These costs are likely to be even lower with mass production. Cassettes can also be made using a black polymer pan that enables closer placement of capillary reaction units and can thus accept a larger number of patient samples per test run. In the future, with a larger Peltier device, an even greater number of samples can be simultaneously tested with rapid multi-target detection. This will enable the higher throughput screening that is required for “on the spot” testing of patients in, for example, STI clinics. Improvements in instrumentation, particularly in thermal regulation, are likely to increase the speed of testing. The technology reported here is urgently needed in the near-POC setting for rapid and meaningful laboratory results to inform an accurate clinical diagnosis followed by appropriate and specific treatment of STIs. This flexible technology can be readily applied to detection of other infectious agents in a clinical setting, for developed and developing countries.
Acknowledgements
This work was funded by the AHFMR Interdisciplinary Team Grants Program managed by Alberta Innovates Health Solutions. We thank Alexander J. Stickel and Mustafa Babadagli for engineering support, Josh Bergman for co-ordinating acquisition of urine samples, and Jahanara Rajwani for lab assistance.References
- World Health Organization, Sexually transmitted infections fact sheet N*110, available: http://www.who.int/mediacentre/factsheets/fs110/en/ 2010 Search PubMed.
- A. M. Foudeh, T. F. Didar, T. Veres and M. Tabrizian, Lab Chip, 2012, 12, 3249–3266 RSC.
- A. Berardi, L. Lugli, C. Rossi, C. L. Maria, I. Guidotti, C. Gallo and F. Ferrari, J. Matern.-Fetal Neonat. Med., 2011, 24, 88–90 CrossRef.
- M. C. Wehrhahn and D. E. Dwyer, Australian Prescriber, 2012, 35, 143–147 Search PubMed.
- J. C. White and S. R. Magee, J. Am. Board Fam. Med., 2011, 24, 758–762 CrossRef.
- O. P. Arya, C. Y. W. Tong, C. A. Hart, B. C. Pratt, S. Hughes, P. Roberts, P. Kirby, J. Howel, A. McCormick and A. D. Goddard, Sex. Transm. Infect., 2001, 77, 58–62 CrossRef CAS.
- M. Salmeri, D. Valenti, S. La Vignera, S. Bellanca, A. Morello, M. A. Toscano, S. Mastrojeni and A. E. Calogero, Journal of Chemotherapy, 2012, 24, 81–86 CrossRef.
- D. Taylor-Robinson and R. F. Lamont, BJOG, 2011, 118, 164–174 CrossRef CAS.
- K. B. Waites, B. Katz and R. L. Schelonka, Clin. Microbiol. Rev., 2005, 18, 757 CrossRef.
- R. N. Fichorova, A. B. Onderdonk, H. Yamamoto, M. L. Delaney, A. M. DuBois, E. Allred and A. Leviton, Mbio, 2011, 2, e00280 Search PubMed.
- E. Juhasz, E. Ostorhazi, K. Ponyai, P. Sillo, L. Parducz and F. Rozgonyi, Reviews in Medical Microbiology, 2011, 22, 73–83 CrossRef.
- S. R. Jangam, A. K. Agarwal, K. Sur and K. D. M., Biosens. Bioelectron., 2013, 42, 69–75 CrossRef CAS.
- X. B. Qiu, D. F. Chen, C. C. Liu, M. G. Mauk, T. Kientz and H. H. Bau, Biomed. Microdevices, 2011, 13, 809–817 CrossRef CAS.
- D. Verdoy, Z. Barrenetxea, J. Berganzo, M. Agirregabiria, J. M. Ruano-Lopez, J. M. Marimon and G. Olabarria, Biosens. Bioelectron., 2012, 32, 259–265 CrossRef CAS.
- X. Y. Pan, L. Jiang, K. Y. Liu, B. C. Lin and J. H. Qin, Anal. Chim. Acta, 2010, 674, 110–115 CrossRef CAS.
- J. Kim, D. Byun, M. G. Mauk and H. H. Bau, Lab Chip, 2009, 9, 606–612 RSC.
- A. Atrazhev, D. P. Manage, A. J. Stickel, H. J. Crabtree, L. M. Pilarski and J. P. Acker, Anal. Chem., 2010, 82, 8079–8087 CrossRef CAS.
- D. P. Manage, J. Lauzon, A. Atrazhev, Y. C. Morrissey, A. L. Edwards, A. J. Stickel, H. J. Crabtree, K. Pabbaraju, G. Zahariadis, S. K. Yanow and L. M. Pilarski, Lab Chip, 2012, 12, 1664–1671 RSC.
- D. P. Manage, L. Chui and L. M. Pilarski, Microfluid. Nanofluid., 2013, 14, 731–741 CrossRef CAS.
- J. Reboud, Y. Bourquin, R. Wilson, G. S. Pall, M. Jiwaji, A. R. Pitt, A. Graham, A. P. Waters and J. M. Cooper, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15162–15167 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c3lc41419a |
| This journal is © The Royal Society of Chemistry 2013 |
