Droplet sorting based on the number of encapsulated particles using a solenoid valve†
Zhenning
Cao
a,
Fangyuan
Chen
b,
Ning
Bao
c,
Huacheng
He
d,
Peisheng
Xu
d,
Saikat
Jana
e,
Sunghwan
Jung
e,
Hongzhen
Lian
b and
Chang
Lu
*fa
aSchool of Biomedical Engineering and Sciences, Virginia Tech-Wake Forest University, Blacksburg, Virginia 24061, USA
bDepartment of Chemistry, Nanjing University, Nanjing, Jiangsu 210032, China
cSchool of Public Health, Nantong University, Nantong, Jiangsu 226019, China
dDepartment of Pharmaceutical and Biomedical Sciences, University of South Carolina, Columbia, South Carolina 29208, USA
eDepartment of Engineering Science and Mechanics, Virginia Tech, Blacksburg, Virginia 24061, USA
fDepartment of Chemical Engineering, Virginia Tech, Blacksburg, Virginia 24061, USA. E-mail: changlu@vt.edu
First published on 19th October 2012
Abstract
Droplet microfluidics provides a high-throughput platform for screening subjects and conditions involved in biology. Droplets with encapsulated beads and cells have been increasingly used for studying molecular and cellular biology. Droplet sorting is needed to isolate and analyze the subject of interest during such screening. The vast majority of current sorting techniques use fluorescence intensity emitted by each droplet as the only criterion. However, due to the randomness and imperfections in the encapsulation process, typically a mixed population of droplets with an uneven number of encapsulated particles results and is used for screening. Thus droplet sorting based on the number of encapsulated particles becomes necessary for isolating or enriching droplets with a specific occupancy. In this work, we developed a fluorescence-activated microfluidic droplet sorter that integrated a simple deflection mechanism based on the use of a solenoid valve and a sophisticated signal processing system with a microcontroller as the core. By passing droplets through a narrow interrogation channel, the encapsulated particles were detected individually. The microcontroller conducted the computation to determine the number of encapsulated particles in each droplet and made the sorting decision accordingly that led to actuation of the solenoid valve. We tested both fluorescent beads and stained cells and our results showed high efficiency and accuracy for sorting and enrichment.
Introduction
Droplet microfluidics focuses on transporting and manipulating monodisperse aqueous droplets within a carrier oil stream in microfluidic devices.1–3 Microfluidic droplets provide uniform microscale compartments that are isolated by the water/oil boundary for conducting reactions4–8 and sorting particles.9–11 Droplet microfluidics has been increasingly used for encapsulating and processing microbeads, cells and molecules for high throughput screening with biological applications.12,13 For example, microbeads have been encapsulated in droplets to create gene encoded protein libraries,14 genomic libraries,15 and conduct single cell genetic analysis.16 Cells may also be encapsulated into droplets, which enable ultrasensitive detection of secreted molecules,13,17 drug screening,10 genetic analysis,18,19 and enzymatic tests.9The generation of droplets with encapsulated particles is an important step for these screening applications. Most encapsulation is based on random loading that produces droplets from particle laden flow.11,13,20–22 Such processes are dictated by Poisson statistics and yields droplets containing uneven number of particles (e.g. with a large fraction of droplets empty when single occupancy is desired). Controlled encapsulation methods have been explored in recent years. Particle alignment offered the potential to generate uniform occupancy beyond Poisson statics.23–25 However, the particle ordering occurs only under specific flow conditions and the encapsulation result could still be affected by the uniformity of the cell distribution throughout the liquid volume. Passive self-sorting of droplets (of 3.5–10 μm diameter) has been demonstrated to separate cell-encapsulated droplets from empty droplets based on the difference in the size.11 Such a scheme does not apply to larger droplets in which the encapsulated particles do not introduce a change in the droplet size. Thus, the majority of the droplet screening systems still need to deal with droplets with inhomogeneous particle occupancy. In contrast, most fluorescence-based droplet detection and sorting systems are based on differentiation of the fluorescence intensity.9,26,27 Droplet sorting methods using the number of encapsulated particles as the criterion are in great need.
In this report, we demonstrate a simple one-layer microfluidic device for droplet sorting based on the number of particles encapsulated. In our device, droplets with encapsulated particles flowed through a narrow interrogation channel where the particles inside were detected by laser-induced fluorescence (LIF) one by one. A programmable microcontroller was used to rapidly process the signal and then make the decision for sorting. We chose to use pulsed pressure generated by an off-chip fast-acting solenoid valve for deflecting selected droplets into the collection outlet. This deflection mechanism avoided complexity in the design and fabrication and potential damage to cell viability associated with other sorting mechanisms such as dielectrophoresis, electrokinetic actuation, surface acoustic waves, piezoelectric force and ultrasound beam.9,28–37 We demonstrated a sorting speed up to ∼30 droplets s−1. We tested both microbeads and cells as the encapsulated particles. We demonstrated highly specific sorting of two-particle droplets from a mixed population and the collection yielded a very high percentage of the targeted droplets (96.3% for microbeads and 93.7% for cells). We envision that our device will find applications mainly in two fronts: 1. Generation of uniform droplet population with the same number of particles encapsulated in each droplet; 2. Analysis of a mixed droplet population that yields information on the particle number in each droplet.
Materials and methods
Microfluidic chip fabrication
The microfluidic droplet sorter was fabricated using a standard soft lithography method as described in our previous publication.38 The microscale patterns were first created using a computer-aided design software (FreeHand MX) and then printed out on high-resolution (5080 dpi) transparencies. The transparencies were used as photomasks in photolithography on a negative photoresist (SU-8 2075, MicroChem, Newton, MA, USA). The thickness of the photoresist and hence the depth of the channels was 50 μm. The pattern of the microfluidic network in the photomask was replicated in SU-8 after exposure and development. The microfluidic chip was molded by casting a layer (about 5 mm thick) of PDMS prepolymer mixture (General Electric silicone RTV 615, MG chemicals) with a mass ratio of A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B = 10
B = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on the photoresist/silicon wafer master. The prepolymer mixture was cured at 85 °C for 2 h in an oven and then peeled off from the master. Glass slides were cleaned in a basic solution (H2O
1 on the photoresist/silicon wafer master. The prepolymer mixture was cured at 85 °C for 2 h in an oven and then peeled off from the master. Glass slides were cleaned in a basic solution (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3 (27%)
NH3 (27%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 (30%) = 5
H2O2 (30%) = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, volumetric ratio) at 75 °C for 1 h and then rinsed with DI water and blown dry. The surfaces of the PDMS chip and a clean glass slide were oxidized using a plasma cleaner (Harrick Plasma, Ithaca, NY, USA). The PDMS chip was then immediately brought into contact against the slide after oxidation to form irreversible bonding. The device was baked at 85 °C for 1 h for further strengthening of the bonding. To render the channel hydrophobic for droplet generation and manipulation, Aquapel (PPG Industries, Pittsburgh, PA, USA) was used to coat the channel before it was blown out of the channel by air.22
1, volumetric ratio) at 75 °C for 1 h and then rinsed with DI water and blown dry. The surfaces of the PDMS chip and a clean glass slide were oxidized using a plasma cleaner (Harrick Plasma, Ithaca, NY, USA). The PDMS chip was then immediately brought into contact against the slide after oxidation to form irreversible bonding. The device was baked at 85 °C for 1 h for further strengthening of the bonding. To render the channel hydrophobic for droplet generation and manipulation, Aquapel (PPG Industries, Pittsburgh, PA, USA) was used to coat the channel before it was blown out of the channel by air.22
Fluorescent beads and cell samples
Fluorescent polystyrene beads with diameter of 16 ± 0.05 μm (s.d.) were purchased from Duke Scientific. The density of beads was 1.05 g ml−1 according to the manufacturer's specification. Before experiments, the beads were diluted and sonicated in DI water at a concentration of 1 × 106/ml in the presence of 5% (v/v) Pluronic F127 (Sigma, St. Louis, MO, USA), which acted as a steric stabilizer of the beads.39 The buffer also contained dextran (Sigma, St. Louis, MO, USA) at a weight/volume fraction of 10% in order to increase the buffer density and prevent beads settling during operation. HeLa (CCL-2) cells were grown at 37 °C with 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) (Mediatech, Herndon, VA) supplemented with 10% (v/v) fetal bovine serum (Sigma) and 1% penicillin (100 μg ml−1, Sigma). The cells were trypsinized and diluted at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5–1
5–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 every 2 d to maintain the cells in the exponential growth phase. The harvested cells were centrifuged at 300 g for 5 min and resuspended in DMEM culture medium at a final concentration of 1 × 106 cells ml−1. The cell suspension was incubated with 2 μM Calcein AM green (Invitrogen, Grand Island, NY, USA) for 30 min at 37 °C for fluorescent labeling.40,41
8 every 2 d to maintain the cells in the exponential growth phase. The harvested cells were centrifuged at 300 g for 5 min and resuspended in DMEM culture medium at a final concentration of 1 × 106 cells ml−1. The cell suspension was incubated with 2 μM Calcein AM green (Invitrogen, Grand Island, NY, USA) for 30 min at 37 °C for fluorescent labeling.40,41
System setup and operation
A schematic of the droplet sorting device is shown in Fig. 1. The microfluidic chip was mounted on an inverted microscope (IX-71, Olympus, Melville, NY, USA) equipped with a 10× dry objective (NA = 0.60). The two inlets of the droplet generation section (one for oil and the other for aqueous particle suspension) (Fig. 1A) were connected to syringe pumps (Fusion 400; Chemyx, Stafford, TX, USA) through perfluoroalkoxyalkane (PFA) tubing (1622L; IDEX Health &Science LLC, Oak Harbor, WA, USA). The inlet of the actuation channel was connected to a pressure source of ∼1 psig via a solenoid valve (ASCO Scientific, Florham Park, NJ, USA) and PFA tubing. Droplets were produced in the T-junction channel as previously described.21 As a result of the shear forces that arose by bringing two immiscible fluids together, the subsequent capillary instability led to generation of monodisperse aqueous droplets in the oil. The continuous phase was of fluorocarbon oil (FC-40, 3 M, St. Paul, MN, USA) containing 5.0% (w/w) PFPE-PEG block-copolymer surfactant used in our previous publication.22 To avoid the hydrophilization of the channel, the continuous phase was first introduced to fill all the channels. Droplet movement could be monitored by a CCD camera (ORCA-285, Hamamatsu, Bridgewater, NJ, USA) or a high speed camera (MotionXtra N3, IDT, Tallahasse, FL, USA) mounted on an eyepiece port of the inverted microscope.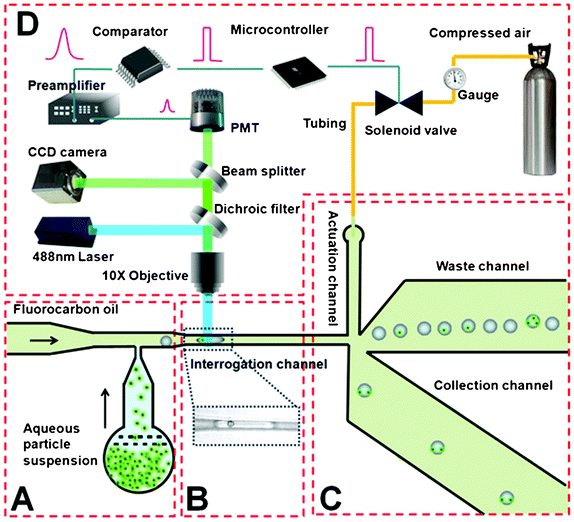 | ||
| Fig. 1 Schematic of the droplet sorting system. All the microfluidic channels have a uniform depth of 50 μm. (A) Droplet generation. The aqueous particle suspension (containing either microbeads or cells) meets the fluorocarbon oil stream at the T junction to produce droplets. The channel widths of aqueous and oil inlets are 30 and 40 μm respectively. (B) Droplet interrogation. The narrow width (∼23 μm) of the interrogation channel allows encapsulated particles to be detected individually (with the image of a droplet containing a single bead shown in the inset). (C) Droplet sorting. The droplets exclusively flow into the waste channel due to the difference in the hydrodynamic resistance (the length of the waste channel is 2 mm while that of the collection channel is 3.4 mm; both channels are 300 μm wide) until they are swept into the collection channel by the pressure pulse from the actuation channel. The actuation channel (80 μm wide and 2 mm long) is connected to a pressure source (∼1 psig) via an off-chip solenoid valve. In the schematic, we suggest selective isolation of droplets that contain two particles from a mixture. (D) Off-chip detection and control system. The fluorescence signal is detected by a photomultiplier tube and further processed by a comparator and a microcontroller. The microcontroller makes the sorting decision and operates the solenoid valve. | ||
A 488 nm laser beam (Cyan-488-50-FP-CDRH, Newport, Franklin, MA) was focused in the narrow interrogation channel by a 10× objective for laser-induced fluorescence detection (Fig. 1B). The intensity of the laser beam was adjusted by a set of neutral density filters (Newport, Franklin, MA). The laser was introduced into the microscope via a laser side port and was reflected by a 505DCLP dichroic beam splitter (Chroma Technology, Bellows Falls, VT, USA) into the 10× objective. The fluorescence emission was collected by the same objective and filtered by a spectral filter (D535/40 emission filter, Chroma Technology). A 20 μm pinhole (P20S, Thorlabs, Newton, NJ, USA) was placed in the optically conjugate plane in front of a photomultiplier tube (R9220, Hamamatsu, Bridgewater, NJ, USA) biased at 550 V, to eliminate out-of-focus signal. The electrical signal generated by the PMT was amplified and filtered by a low noise current preamplifier (SR570, Standard Research System, Sunnyvale, CA, USA) for optimum signal-to-noise ratio (SNR). The signal was then processed by a differential comparator (TL712, Texas Instrument, Dallas, TX, USA) before input into a microcontroller (AT89S51, Atmel, San Jose, CA, USA). When a target droplet was detected, the microcontroller activated a reed relay (R56-1D.5-6D, NTE Electronics, Bloomfield, NJ, USA), which in turn operated the solenoid valve and thus diverted the target droplet into the collection channel. Both the fluorescence analog signal out of the preamplifier and converted digital signal out of the comparator could be examined or recorded by an oscilloscope (B&K precision2530, Newark, Chicago, IL, USA) or a PCI data acquisition card (PCI- 6254, National Instruments, Austin, TX, USA).
Results and discussion
The microfluidic device, as shown in Fig. 1, has a simple one-layer structure. We used a T-junction (Fig. 1A) to produce droplets with encapsulated particles, having the carrier fluorocarbon oil and the aqueous particle (microbead or cell) suspension coming from separate inlets. The generated droplets were directed into a narrow interrogation channel, which was only 23 μm wide in order to ensure the vast majority of encapsulated particles were detected individually in the laser focal volume42 (∼15 μm in the diameter, generated by a 488 nm laser focused through a 10× objective) (Fig. 1B). Droplets were sorted in the downstream where the interrogation channel met with three other channels (i.e. the actuation channel, the waste channel and the collection channel) (Fig. 1C).Without pulsed pressure from the actuation channel, the difference in the hydrodynamic resistance resulted from the different channel lengths (the waste and collection channels were 2 and 3.4 mm long, respectively) drove droplets exclusively into the waste channel. This directional motion can be explained by Zweifach–Fung effect, according to which the asymmetric distribution of pressure gradient and shear forces on the surface of a droplet draw it towards the branch with higher flow rate.43 The waste and collection channels had large widths (∼300 μm) in order to decrease the impact of the droplets on the hydrodynamic resistance.44–46 The actuation channel was connected to a pressure source (∼1 psig) via an off-chip fast-acting solenoid valve operated by a microcontroller. As the fluorescence emitted by encapsulated particles was collected and processed by the optical detection and signal processing system (Fig. 1D), the microcontroller made the decision about sorting to the collection channel in the case of detecting target droplets (further detailed below). When actuated, the solenoid valve was rapidly switched open and then shut, creating a pulsed pressure (∼1 psig) with a duration of 7 ms. As we show in the COMSOL simulation (Video 1, ESI†), the resultant fluid pressure would sweep the droplet (exiting the interrogation channel) into the collection branch. Our simulation shows that the entire sorting process would take less than 8 ms until the flow restores to its original state. With a response time of roughly 10 ms for the solenoid valve used (based on the manufacturer specification), our system has the potential of sorting up to 55 Hz. It is worth noting that the fastest solenoid valves these days may have response time as short as 1 ms and thus would offer a much higher sorting rate. The use of solenoid valves for microfluidic sorting makes the device design very simple. Furthermore, such an arrangement is entirely compatible with a large number of integrated microfluidic systems because solenoid valves are widely used for two-layer valve switching.47–49
We established the robustness of the solenoid valve sorting by demonstrating simple separation of droplets with and without fluorescence. We first examined a mixed population of fluorescent and nonfluorescent droplets generated in the upstream of the sorter by a previously published method.50 In order to facilitate differentiation under phase contrast microscopy, the fluorescent droplets contained fluorescein and the nonfluorescent droplets were doped with trypan blue dye. As shown in Fig. 2a, the sorter was able to deliver the nonfluorescent droplets into the waste channel (left) and the fluorescein-containing droplets into the collection channel (right). In general, the accuracy was very high for such sorting. In Fig. 2b, we show that the sorting accuracy remained at 97.5% even we increased the droplet throughput to 30 droplets s−1. Furthermore, we demonstrate that droplets with encapsulated fluorescent particles can be separated from droplets without particles. As shown in Fig. 2c, the empty droplets were directed into the waste channel and the droplets with fluorescent polystyrene beads (D ∼16 μm) were sorted into the collection channel. The sorting accuracy in this case, varying from 97% at 5 droplets s−1 to 90% at 30 droplets s−1 (Fig. 2d), was slightly lower than that of sorting fluorescein droplets (Fig. 2b). This was due to missed detection of encapsulated particles when the small laser focal volume did not fully cover the space occupied by the particle(s). Thus the mistaken sorting events were all false negatives (i.e. taking droplets with fluorescent beads as those without beads). Finally, we also tested droplets with encapsulated cells (Calcein AM stained HeLa cells), as shown in Fig. 2e and f. Culture medium instead of DI water was used in the aqueous phase and a block-copolymer surfactant was added to the continuous oil phase to stabilize the droplets while keeping cell viability.22 The sorting accuracy of 95.1% at 5 droplets s−1 and 86.9% at 30 droplets s−1 for cell-containing droplets (Fig. 2f) is slightly lower than that of sorting bead-containing droplets (Fig. 2d). We attribute the difference to the uneven size and fluorescence intensity from individual cells. Despite this, the accuracy is higher than most of previously described microfluidic FACS sorters.51,52 Similar to the bead-containing droplet sorting, there were only false negatives and a high purity of cell-containing droplets (100%) was obtained in the collection end.
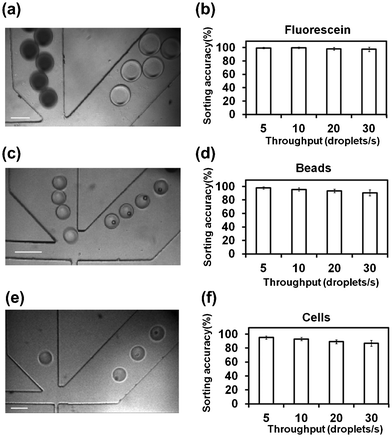 | ||
| Fig. 2 Droplet sorting based on emitted fluorescence. CCD camera images confirm that droplets containing (a) fluorescein; (c) fluorescent beads; (e) fluorescently stained HeLa cells were sorted into the collection channel (right). The scale bar is 100 μm. The sorting accuracy (i.e. the percentage of droplets being correctly sorted) in these three cases: (b) fluorescein; (d) fluorescent beads; (f) fluorescently stained HeLa cells, at various droplet throughputs (5–30 droplets s−1) is also shown. The flow rates of the aqueous phase and the oil phase were varied in the ranges of 0.05–0.5 and 2–10 μl min−1, respectively, in the experiment to achieve different droplet throughputs. The droplet diameter ranged from 30 to 120 μm (viewed in the channel). The experiments were conducted in triplicate. For each run, at least 2000 droplets were observed for calculating of the sorting accuracy. | ||
In order to sort droplets based on the number of encapsulated particles, we first examined whether our system generated a reliable signal that would indicate the number of particles in each droplet. As shown in Fig. 3a, typical fluorescence signals detected from a mixed droplet population with encapsulated fluorescent beads presents peaks of various intensities. As shown in the zoomed-in spectrum in Fig. 3a, each droplet generates a broad peak due to the light scatter at the water/oil interface. The broad peaks may have one or two embedded high-intensity peaks that were produced by encapsulated fluorescent beads. The variance in the peak intensity was likely to be due to the randomness in the bead position when each bead passed through the laser focal volume. Similar data were also generated by cell-containing droplets (Fig. 3c). In general, the raw fluorescence peaks generated by encapsulated cells showed even more variance than beads, due to the non-uniform cell size. Nevertheless, we could use a custom MATLAB program to extract information on the number of particles in each droplet and then generate the occupancy distributions (Fig. 3b and d). The occupancy distributions produced by both fluorescent beads and stained cells can be fit by the Poisson distribution very well. Because the particle occupancy of droplets is known to be governed by the Poisson distribution,9,11,13,20 the agreement between the experimental data and the predicted distribution confirms the system's ability to accurately detect the vast majority of the encapsulated particles.
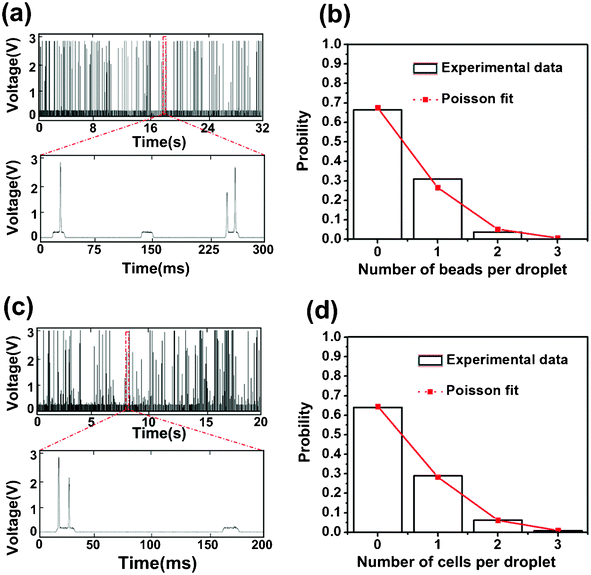 | ||
| Fig. 3 The fluorescence signal contains information on the number of encapsulated particles per droplet. The fluorescence intensity data over time are shown for droplets with encapsulated (a) fluorescent beads and (c) fluorescently stained HeLa cells. The flow rates were set at 0.15 and 3.6 μl min−1 for the aqueous and oil phase, respectively. Using the MATLAB program, we extracted the particle number per droplet from these fluorescence intensity data. The probability distribution in terms of the particle number per droplet obtained from these experimental data for (b) fluorescent beads and (d) fluorescently stained HeLa cells (by analyzing droplet populations >2000) fits the Poisson distribution (delineated in red line). Poisson distribution is defined by P(k) = λke−λ/k!, where k is the number of particle per droplet (0–3) and λ is the average number of particles per droplet (0.39 for beads, 0.44 for cells). | ||
We constructed sophisticated signal processing system including a comparator and a microcontroller for extracting information on the number of particles in each droplet and making a sorting decision accordingly, all in real time. We used a comparator to compare the detected fluorescence signal (in analog voltage) with a threshold voltage and the comparator output a constant voltage over the period that the fluorescence signal was higher than the threshold (the output is zero during other times) (Fig. S1, ESI†). With the preset threshold (higher than the broad peak intensity), the broad peaks generated by light scatter were filtered out and the high-intensity peaks were converted to peaks of uniform height (Fig. 4). The digitalized peaks (Fig. 4b) were then input into a microcontroller. A microcontroller is a small computer on a single integrated circuit that is often used in automatically controlled systems. In our case, the microcontroller did the computation to determine two facts: 1. Are the consecutive particles encapsulated in the same droplet? 2. How many particles are in a droplet? Fig. 5 shows the algorithm used to sort specifically droplets that contain two particles. The algorithm determines whether multiple particles are within the same droplet based on the interval between the particles. When the particles are in the same droplet, the interval is shorter than the time required for a droplet to pass through the detection point, assuming that the distance between droplets was significantly longer than the droplet length. The algorithm also included a counter to register the number of particles in one droplet.
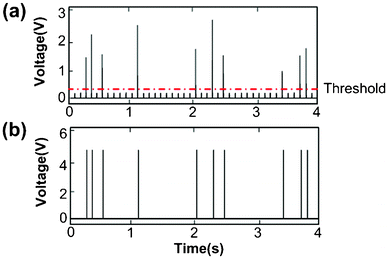 | ||
| Fig. 4 The processing of the fluorescence intensity data by the comparator. The data were generated using droplets with encapsulated fluorescent beads and flow rates of 0.15 and 3.6 μl min−1 for the aqueous and oil phases, respectively. (a) The raw fluorescence intensity data over time before the processing. A threshold of 0.35 V is set for determining which peaks are generated by fluorescent beads. (b) The signal after the comparator processing. All peaks higher than the threshold are converted to peaks of uniform intensity (5 V). | ||
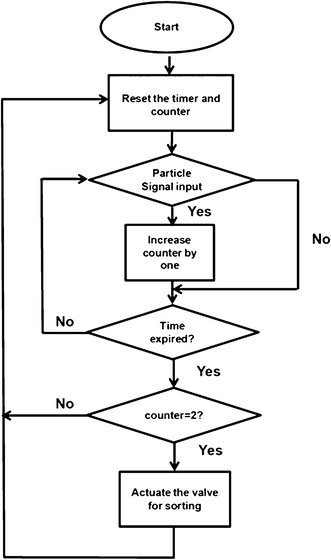 | ||
| Fig. 5 A flowchart that summarizes the logic of the microcontroller for sorting two-particle droplets out of a mixed population. A timer and a counter are needed to determine how many particles are in one droplet. The droplet transit time (i.e. the time required for a droplet to travel through the laser detection point) was used as a parameter to determine whether multiple particles are encapsulated in the same droplet. If the peaks are detected within one unit of the transit time, they are considered in the same droplet. In the interrogation channel, the distance between two droplets was significantly longer than the droplet length. The counter registers the number of the particles in a droplet. | ||
We used our microfluidic sorter to specifically sort two-particle droplets out of a mixed population. Fig. 6 shows that such sorting could be successfully applied to droplets containing fluorescent beads or stained HeLa cells. The two-bead/two-cell droplets were sorted into the collection channel, while all other types of droplets (i.e. droplets containing 0, 1, 3 or 4 particles) were directed into the waste channel at a throughput of ∼10 droplets s−1. The videos that recorded such sorting are included as Videos 2 and 3 in ESI†. We used the images taken by the CCD camera to independently verify the sorting efficiency (Note: the use of the camera is not required for sorting) and the results are shown in Table 1. The two-bead droplets and two-cell droplets from a mixed population could be highly enriched after a single pass through the sorter. Whereas the initial percentage of two-bead droplets in the starting population was 5%, the collection channel ended up having 96% two-bead droplets and the waste had only 1% two-bead droplets. Similarly, two-cell droplets were enriched from an initial concentration of 6% to 93% in the collection reservoir. The errors could be attributed to the fact that beads or cells might stick to or overlay on each other while passing the detection point. In principle, further decrease in the size of the interrogation channel could increase the accuracy. However, the practice may also increase the risk of clogging. Thus, a balance between the sorting accuracy and the system robustness needs to be reached.
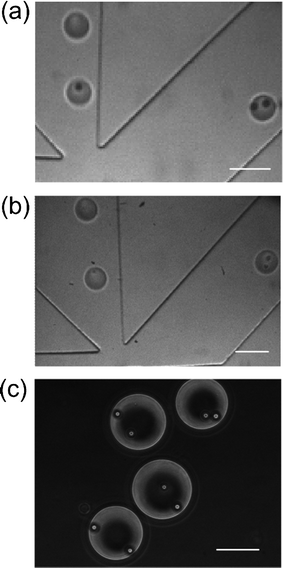 | ||
| Fig. 6 Camera images show that only two-particle droplets were sorted out from a mixed population. The droplets containing 0, 1, 3 particles flow into the waste channel (left) and droplets containing (a) two beads or (b) two cells are deflected into the collection channel (right). The droplets were generated with flow rates of 0.15 μl min−1 for the aqueous phase and 3.60 μl min−1 for the oil phase. (c) Uniformly two-bead droplets in the outlet reservoir of the collection channel. The droplets were generated under the flow rates of 0.27 μl min−1 for the aqueous phase and 2.10 μl min−1 for the oil phase. The scale bars represent 100 μm. | ||
| Occupancy | Bead-containing droplets | Cell-containing droplets | ||||||
|---|---|---|---|---|---|---|---|---|
| Input | Collection | Input | Collection | |||||
| Number | Percentage (%) | Number | Percentage (%) | Number | Percentage (%) | Number | Percentage (%) | |
| 0 | 1459 | 68.4 | 0 | 0 | 1145 | 64.0 | 0 | 0 |
| 1 | 560 | 26.2 | 0 | 0 | 520 | 29.0 | 0 | 0 |
| 2 | 101 | 4.7 | 79 | 96.3 | 104 | 5.8 | 74 | 93.7 |
| 3 | 12 | 0.6 | 3 | 3.7 | 17 | 0.95 | 5 | 6.3 |
| 4 | 0 | 0 | 0 | 0 | 3 | 0.002 | 0 | 0 |
Acknowledgements
We acknowledge funding from National Science Foundation CBET 1016547, 0967069 and the New Investigator Awards Program from the American Association of College of Pharmacy (AACP).References
- S. Y. Teh, R. Lin, L. H. Hung and A. P. Lee, Lab Chip, 2008, 8, 198–220 RSC.
- A. Huebner, S. Sharma, M. Srisa-Art, F. Hollfelder, J. B. Edel and A. J. Demello, Lab Chip, 2008, 8, 1244–1254 RSC.
- M. Joanicot and A. Ajdari, Science, 2005, 309, 887–888 CrossRef CAS.
- B. Zheng, L. S. Roach and R. F. Ismagilov, J. Am. Chem. Soc., 2003, 125, 11170–11171 CrossRef CAS.
- M. R. Bringer, C. J. Gerdts, H. Song, J. D. Tice and R. F. Ismagilov, Philos. Trans. R. Soc. London, Ser. A, 2004, 362, 1087–1104 CrossRef CAS.
- H. Song, D. L. Chen and R. F. Ismagilov, Angew. Chem., Int. Ed., 2006, 45, 7336–7356 CrossRef CAS.
- A. Günther and K. F. Jensen, Lab Chip, 2006, 6, 1487–1503 RSC.
- Z. Y. Han, W. T. Li, Y. Y. Huang and B. Zheng, Anal. Chem., 2009, 81, 5840–5845 CrossRef CAS.
- J. C. Baret, O. J. Miller, V. Taly, M. Ryckelynck, A. El-Harrak, L. Frenz, C. Rick, M. L. Samuels, J. B. Hutchison and J. J. Agresti, Lab Chip, 2009, 9, 1850–1858 RSC.
- E. Brouzes, M. Medkova, N. Savenelli, D. Marran, M. Twardowski, J. B. Hutchison, J. M. Rothberg, D. R. Link, N. Perrimon and M. L. Samuels, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 14195–14200 CrossRef CAS.
- M. Chabert and J. L. Viovy, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 3191 CrossRef CAS.
- Y. C. Tan, K. Hettiarachchi, M. Siu, Y. R. Pan and A. P. Lee, J. Am. Chem. Soc., 2006, 128, 5656–5658 CrossRef CAS.
- J. Clausell-Tormos, D. Lieber, J. C. Baret, A. El-Harrak, O. J. Miller, L. Frenz, J. Blouwolff, K. J. Humphry, S. Köster and H. Duan, Chem. Biol., 2008, 15, 427–437 CrossRef CAS.
- A. D. Griffiths and D. S. Tawfik, EMBO J., 2003, 22, 24–35 CrossRef CAS.
- T. Kojima, Y. Takei, M. Ohtsuka, Y. Kawarasaki, T. Yamane and H. Nakano, Nucleic Acids Res., 2005, 33, e150–e150 CrossRef.
- Y. Zeng, R. Novak, J. Shuga, M. T. Smith and R. A. Mathies, Anal. Chem., 2010, 82, 3183–3190 CrossRef CAS.
- J. Q. Boedicker, M. E. Vincent and R. F. Ismagilov, Angew. Chem., Int. Ed., 2009, 48, 5908–5911 CrossRef CAS.
- R. Novak, Y. Zeng, J. Shuga, G. Venugopalan, D. A. Fletcher, M. T. Smith and R. A. Mathies, Angew. Chem., Int. Ed., 2011, 50, 390–395 CrossRef CAS.
- H. Zhang, G. Jenkins, Y. Zou, Z. Zhu and C. J. Yang, Anal. Chem., 2012, 84, 3599–3606 CrossRef CAS.
- S. Köster, F. E. Angile, H. Duan, J. J. Agresti, A. Wintner, C. Schmitz, A. C. Rowat, C. A. Merten, D. Pisignano and A. D. Griffiths, Lab Chip, 2008, 8, 1110–1115 RSC.
- Y. Zhan, J. Wang, N. Bao and C. Lu, Anal. Chem., 2009, 81, 2027–2031 CrossRef CAS.
- F. Chen, Y. Zhan, T. Geng, H. Lian, P. Xu and C. Lu, Anal. Chem., 2011, 83, 8816–8820 CrossRef CAS.
- J. F. Edd, D. Di Carlo, K. J. Humphry, S. Köster, D. Irimia, D. A. Weitz and M. Toner, Lab Chip, 2008, 8, 1262–1264 RSC.
- A. R. Abate, C. H. Chen, J. J. Agresti and D. A. Weitz, Lab Chip, 2009, 9, 2628–2631 RSC.
- E. W. Kemna, R. M. Schoeman, F. Wolbers, I. Vermes, D. A. Weitz and A. van den Berg, Lab Chip, 2012, 12, 2881–2887 RSC.
- J. J. Agresti, E. Antipov, A. R. Abate, K. Ahn, A. C. Rowat, J. C. Baret, M. Marquez, A. M. Klibanov, A. D. Griffiths and D. A. Weitz, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 4004–4009 CrossRef CAS.
- K. Bernath, M. Hai, E. Mastrobattista, A. D. Griffiths, S. Magdassi and D. S. Tawfik, Anal. Biochem., 2004, 325, 151–157 CrossRef CAS.
- B. Ahn, K. Lee, R. Louge and K. W. Oh, Biomicrofluidics, 2009, 3, 044102 CrossRef.
- K. Ahn, C. Kerbage, T. P. Hunt, R. Westervelt, D. R. Link and D. Weitz, Appl. Phys. Lett., 2006, 88, 024104 CrossRef.
- P. S. Dittrich and P. Schwille, Anal. Chem., 2003, 75, 5767–5774 CrossRef CAS.
- T. Franke, S. Braunmüller, L. Schmid, A. Wixforth and D. Weitz, Lab Chip, 2010, 10, 789–794 RSC.
- L. Johansson, F. Nikolajeff, S. Johansson and S. Thorslund, Anal. Chem., 2009, 81, 5188–5196 CrossRef CAS.
- C. Lee, J. Lee, H. H. Kim, S. Y. Teh, A. Lee, I. Y. Chung, J. Y. Park and K. K. Shung, Lab Chip, 2012, 12, 2736–2742 RSC.
- C. B. Rohde, F. Zeng, R. Gonzalez-Rubio, M. Angel and M. F. Yanik, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 13891 CrossRef CAS.
- J. Shemesh, A. Bransky, M. Khoury and S. Levenberg, Biomed. Microdevices, 2010, 12, 907–914 CrossRef.
- J. Shi, H. Huang, Z. Stratton, Y. Huang and T. J. Huang, Lab Chip, 2009, 9, 3354–3359 RSC.
- N. Sundararajan, D. Kim and A. A. Berlin, Lab Chip, 2005, 5, 350–354 RSC.
- J. Wang, Y. Zhan, V. M. Ugaz and C. Lu, Lab Chip, 2010, 10, 2057–2061 RSC.
- S. Moghimi, Adv. Drug Delivery Rev., 1995, 16, 183–193 CrossRef CAS.
- H. Y. Wang and C. Lu, Chem. Commun., 2006, 3528–3530 RSC.
- H. Y. Wang and C. Lu, Anal. Chem., 2006, 78, 5158–5164 CrossRef CAS.
- M. Srisa-Art, I. C. Bonzani, A. Williams, M. M. Stevens and J. B. Edel, Analyst, 2009, 134, 2239–2245 RSC.
- V. Doyeux, T. Podgorski, S. Peponas, M. Ismail and G. Coupier, J. Fluid Mech., 2011, 674, 359 CrossRef CAS.
- T. Glawdel, C. Elbuken and C. Ren, Lab Chip, 2011, 11, 3774–3784 RSC.
- F. Jousse, R. Farr, D. R. Link, M. J. Fuerstman and P. Garstecki, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2006, 74, 036311 CrossRef.
- W. Engl, M. Roche, A. Colin, P. Panizza and A. Ajdari, Phys. Rev. Lett., 2005, 95, 208304 CrossRef.
- A. Y. Fu, H. P. Chou, C. Spence, F. H. Arnold and S. R. Quake, Anal. Chem., 2002, 74, 2451–2457 CrossRef CAS.
- M. A. Unger, H. P. Chou, T. Thorsen, A. Scherer and S. R. Quake, Science, 2000, 288, 113–116 CrossRef CAS.
- T. Thorsen, S. J. Maerkl and S. R. Quake, Science, 2002, 298, 580–584 CrossRef CAS.
- L. H. Hung, K. M. Choi, W. Y. Tseng, Y. C. Tan, K. J. Shea and A. P. Lee, Lab Chip, 2006, 6, 174–178 RSC.
- C. H. Chen, S. H. Cho, F. Tsai, A. Erten and Y. H. Lo, Biomed. Microdevices, 2009, 11, 1223–1231 CrossRef CAS.
- M. M. Wang, E. Tu, D. E. Raymond, J. M. Yang, H. Zhang, N. Hagen, B. Dees, E. M. Mercer, A. H. Forster and I. Kariv, Nat. Biotechnol., 2004, 23, 83–87 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2lc40950j |
| This journal is © The Royal Society of Chemistry 2013 |
