Generation of monodispersed microdroplets by temperature controlled bubble condensation processes†
Kai
Wang
,
Lisi
Xie
,
Yangcheng
Lu
* and
Guangsheng
Luo
*
The State Key Lab of Chemical Engineering, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China. E-mail: luyc@mail.tsinghua.edu.cn; gsluo@mail.tsinghua.edu.cn
First published on 22nd October 2012
Abstract
This work introduces a microfluidic method for the generation of monodispersed microdroplets by using temperature controlled bubble condensation processes. In this method, the dispersed phase is first vaporized in the feeding pipe and ruptured to monodispersed bubbles in a coflowing stream. These bubbles are then condensed in the downstream pipe, where monodispersed microdroplets are obtained. This method ensures the narrow distribution of droplet diameters and prepares microdroplets less than 200 μm in sub-millimeter fluidic devices.
1. Introduction
Micrometer scaled droplets with a narrow size distribution have drawn considerable attention from researchers in the last decade.1,2 These droplets have some extremely important characteristics, such as small sample volumes,3 fast reagent transfer rates,4 low testing costs and high analysis efficiencies.5 The uniform droplet size ensures the experimental accuracy and repeatability, which is important in chemical synthesis,6 bio-analysis,7 interfacial science8 and materials preparation.9,10Microfluidic technology is one of the most important methods for the generation of microdroplets. Size regulated microdroplets with a relative diameter deviation of less than 5% have been successfully produced in T-junction, flow focusing, co-axial, as well as other types of microfluidic generators.11–13 The generation method and droplet size is determined by several parameters, including channel hydraulic diameter, capillary number, Weber number, two-phase flow rate ratio and viscosity ratio.11,14 To obtain smaller microdroplets, the most effective method is using low hydraulic diameter channels, since the dimensionless droplet diameters (d/w, w is the channel width) always range between 0.5 to 5 in most microfluidic devices.15–18 However, this is not always the best choice for specific applications due to the difficulty and high cost of fabrication and the ease of clogging for a channel smaller than the sub-millimeter scale. Researchers have to balance the fabrication, operation and task demands when choosing their microfluidic structures for experiments. Usually, the hydraulic diameters of microchannels range from 20 to 200 micrometers.19–21
Using a millimeter or sub-millimeter scaled channel it is very hard to obtain microscaled droplets with volumes on the nanoliter or picoliter scale. To reduce droplet size, the device must operate at a high capillary number to produce an adequate shearing force. In these processes, surfactants are important for reducing the system interfacial tension (γ < 10 mN m−1).8,22 High continuous phase viscosities (μ > 10 mPa·s) and shearing velocities (u > 0.1 m s−1) are commonly used15,23 as well. According to some studies on the microdroplet break-up mechanism, it is also very easy to form jetting flow patterns at high continuous phase capillary numbers (Ca = μu/γ > 0.1).24,25 Since the jetting fluid is ruptured under the Rayleigh–Plateau instability and satellite droplets usually form in the jetting flow pattern,19,24,26 it is difficult to generate uniform droplets in the jetting flow regime.
In this work, we introduce a new monodispersed microdroplet generation method by condensing monodispersed bubbles generated in sub-millimeter scaled fluidic devices. This method is appropriate for low boiling point fluids and avoids jetting flow by using gas/liquid dispersion processes. In this method, the liquid dispersed phase is first vaporized to a gas phase and then ruptured to form uniform bubbles in the continuous phase. The bubble generating process proceeds at an operating temperature higher than the boiling point of the dispersed phase. Finally, the uniform bubbles condense into uniform droplets by reducing the operating temperature. This method is credible and versatile enough for wide application to processes involving the continuous production of microdroplets.
2. Experiment
The experiments were carried out in a microfluidic system, as shown in Fig. 1. The system is organized by four individual units, including fluid feeding, heating, cooling and monitoring, which are connected with Teflon tubing (inner diameter is 0.5 mm). Two microscopes equipped with transmitted light sources are placed on the heating and monitoring units to capture the formed bubbles and droplets with cameras (PL-A741, PixeLINK). In the feeding unit the two phases are driven by two syringe pumps (LSP02-1B, Longer) and gas-tight syringes (0.25 mL, 1 mL, 10 mL, Shanghai Gaoge). The dispersed phase is vaporized in a mini-evaporator, the detailed structure of which is given by Fig. 2a. The mini-evaporator heated by a ceramic heating plate (220 V, 100 W) had a surface temperature of nearly 70 °C (measured with an infrared temperature sensor). The vapor temperature outside of the mini-evaporator was 1 or 2 degrees higher than 40 °C, measured from the metal pipe connected to the evaporator outlet. The continuous phase was heated by two stainless steel coiled pipes in the heating unit. To ensure the gas/liquid dispersion process, the ambient temperature in the heating unit was controlled with a self-made air bath. Fig. 2b gives a schematic diagram of the air bath. The atmosphere in the air bath was heated by its metal shell and two radiators (220 V, 100 W). A commercial temperature sensor (Pt-100) was placed in the air bath to collect the temperature data. This sensor was placed near the microchip and connected to a PID (proportion–integration–differentiation) controller to make the heating unit work periodically. This air bath was placed on the microscope platform and maintained an operating temperature of 40 ± 1.5 °C during experiments. The bubble generators in the air bath were fabricated from PMMA (polymethyl methacrylate) plates with end mills and sealed with a high-pressure thermal sealing device (A274, Techson) at 75 °C, 0.4 MPa. As shown in Fig. 2c, some tapered glass capillaries are embedded in these generators to form coflowing dispersion structures. The main channels of the generators were designed as square and round sections with different hydraulic diameters (three generators named S1, W = 800 μm; S2, W = 600 μm and S3, D = 300 μm). Wide measurement channels (2 mm width and 1 mm depth) were placed behind the main channels to observe the formed bubbles. Compared with the heating unit, the cooling unit was relatively simple. Ice packs at 0 °C were placed around the Teflon pipes to cool the gas/liquid two phases, as shown in Fig. 2d. The formed microdroplets are observed and recorded in the monitoring unit where a PMMA chip with 600 μm width and 300 μm height channel was placed inside. The supplementary figure (ESI†) gives some pictures of this temperature controlled microfluidic system. | ||
| Fig. 1 A schematic diagram of the microfluidic system used in this work. The heating unit is shown with a red background and the cooling unit is represented with a blue background. | ||
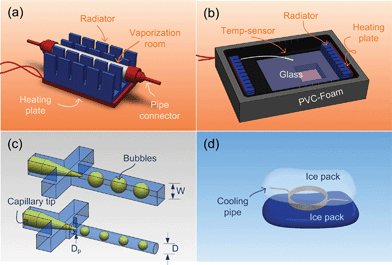 | ||
| Fig. 2 Main components of the temperature controlled microfluidic system. (a) The mini-evaporator fabricated from a stainless steel pipe has 4 mm outer diameter and 3 mm inner diameter. (b) The air bath (bottom-up) fabricated with anodized aluminum has a 160 mm × 120 mm × 40 mm inner space. A glass window is placed on the metal shell to observe the inside. (c) The capillary embedded coflowing generators with outer and inner diameters at 150 μm and 85 μm. (d) The cooling unit with 2 m long cooling pipe. | ||
A low boiling point alkane – n-pentane (BP: 36.1 °C) – was used as the dispersed phase fluid to demonstrate the idea in this work. The continuous phase fluid was an aqueous solution containing 0.5% wt SDS (sodium dodecyl sulfate) and 1% wt PVA (polyvinyl alcohol). The water phase was first heated to 90 °C and then cooled down to room temperature before the experiment to expel soluble gases. The viscosity of the continuous phase was 2.0 mPa·s at 40 °C measured with an Ubbelohde viscometer. The surface tension of pentane (gas)/SDS-PVA solution was 29.2 mN m−1 at 40 °C was measured with a commercial interfacial tensiometer (OCAH200, DataPhysics Instruments GmbH). A control group experiment at room temperature (25 °C) was made to test the size variation of formed microbubbles. This experiment was performed with propane as the dispersed phase and 3% wt SDS aqueous solution as the continuous phase. The viscosity of 3% wt SDS aqueous solution was 0.97 mPa·s and the interfacial tension between propane and SDS solution was 30.9 mN m−1. The n-pentane, SDS and PVA were provided by the Sinopharm Chemical Reagent Co., Ltd (China) and the propane was provided by the Huayuan Gas Co., Ltd (China).
The heating–cooling experiment was started by turning on the heating unit. About 10 min later, n-pentane was pumped into the generators. When the gas flow rate became stable, the water phase was fed in. It took about 15 min for the whole system to re-stabilize. In the control group experiment, propane was first stored in a gas-tight syringe and then pumped into the bubble generator directly. The bubble and droplet diameters were measured from recorded pictures in the wide measurement channels. At least 50 bubbles and 50 droplets were counted in each test to get their average diameters (d = Σdi/n, n is the sample number) and polydispersity indexes (σ = δ/d × 100%, δ is the standard deviation of droplet diameters).
3. Results and discussion
Fig. 3 gives some representative pictures. According to our observation, the vaporized n-pentane was ruptured as short slugs or round bubbles in the PMMA generators. These slugs and bubbles were both ruptured in the dripping flow pattern.15 No matter what shapes of bubbles were generated, only round bubbles were observed in the wide measurement channels due to the large flowing spaces. Although sometimes the flowing bubbles contacted with each other, no coalescence was observed owing to protection of ionic surfactant SDS. Fig. 4 gives the average bubble diameters in different generators. Considering the shearing mechanism of the dripping rupture method, the capillary number of the continuous phase (CaW = μWQW/sγ, where μW is the viscosity of water phase, QW is the flow rate of water phase, s is the section area of the main channels in the generators, s = W2 or πD2/4, and γ is the interfacial tension) determines the variation of bubble size. In the cooling unit, the n-pentane condensed to a liquid phase and flowed out as microdroplets. Fig. 3d and e give some pictures of the formed droplets. The average diameters of bubbles and droplets are given in Fig. 5. Although the highest capillary number of the continuous phase was less than 0.005 in the experiments, the formed droplets are very small, ranging from 43 μm to 200 μm, similar to the droplets generated in 100 μm width channels.27 A simple relation between the bubble diameter and the droplet diameter is correlated from the experimental result as,| ddroplet ≈ 0.24dbubble | (1) |
 | ||
| Fig. 3 Some pictures recorded in the experiment. (a) Bubble generator G1. (b) Bubble generator G3, where a glass capillary with inner diameter 300 μm is embedded in the PMMA channel. (c) The formed bubbles in the wide measurement channel in generator G2. (d) Original and labeled microdroplets condensed from the bubbles in Fig. 3a.; (e) Original and labeled microdroplets condensed from the bubbles in Fig. 3b. Scale bar = 1 mm. | ||
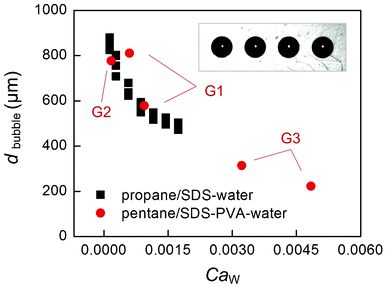 | ||
| Fig. 4 The average bubble diameters. The square dots represent the bubbles generated in G2 with propane as the gas phase, whose flow rates are varied between 30 μL min−1 and 300 μL min−1. The round dots are the average diameters of n-pentane bubbles in different generators. The flow rates of n-pentane (liquid state) are varied from 0.33 μL min−1 to 5 μL min−1. | ||
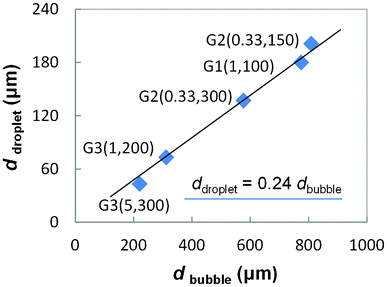 | ||
| Fig. 5 Relation of the average bubble diameter and the average droplet diameter. The numbers in the brackets are the flow rates of the oil phase (liquid state) and water phase controlled by syringe pumps in the experiment (QO, Qw). Both flow rate units are μL min−1. | ||
This relation represents that the volume of the dispersed fluid reduced more than 70 times in the cooling process.
The uniformities of bubble and droplet diameters in this work are characterized by the polydispersity index, which is usually used in emulsion studies.28 All the polydispersity indexes of bubbles and droplets are less than 5%, as shown in Table 1, indicating that monodispersed bubbles and droplets were successfully produced. The droplet generation frequencies ranged from 1.3 Hz to 2000 Hz in the experiment, which is not very high compared with laser and electric field enhanced droplet generation processes.29,30 This generation frequency is mainly confined by the heating power of the mini-evaporator in the experiment, but does not mean this temperature controlled condensing method cannot realize higher droplet generation frequency. According to the analysis from Gunther and Jensen, the bubble and slug flow regions mainly exist as the gas phase superficial velocity lower than 1 m s−1,14 representing nearly 8 mL min−1 of gas flow rate in this work's Teflon tubes. In our other experiment the flow rate of gas phase has reached 5–10 mL min−1 in the similar coflowing air bubble dripping process, showing the droplet generation frequency in this work's sub-millimeter scaled generator can still be increased 10 times.
| G (QO, Qw) | Bubbles | Droplets |
|---|---|---|
| G1 (1, 100) | 1.3% | 2.4% |
| G2 (0.33, 150) | 1.9% | 2.2% |
| G2 (0.33, 300) | 0.8% | 2.9% |
| G3 (1, 200) | 1.1% | 4.0% |
| G3 (5, 300) | 1.6% | 3.8% |
4. Conclusion
In this work, we described a temperature controlled microfluidic method for the preparation of micrometer scaled droplets using sub-millimeter devices. This method ensures the narrow distribution of droplet diameters due to the highly uniform microbubbles produced in the dripping regime, and can notably reduce the viscosity and shearing velocity requirements for the continuous phase. This versatile method has strong potential for the continuous production of microdroplets. Besides producing micrometer droplets, this temperature controlled condensing method also has the potential to be applied to other areas. Further works involving gathering trace organic compounds in water samples and preparing uniform polymer microbeads will be carried out using this bubble condensation method.Acknowledgements
We would like to acknowledge the support of the National Natural Science Foundation of China (21036002, 21106076 and 21176136) and the Postdoctoral Science Foundation of China (20100480283, 201104095) for this work. We acknowledge the help from Mr. Chris P. Tostado for the language revision of this paper.References
- C. X. Zhao and A. Middelberg, Chem. Eng. Sci., 2011, 66, 1394–1411 CrossRef CAS.
- N. Lorber, F. Sarrazin, P. Guillot, P. Panizza, A. Colin, B. Pavageau, C. Hany, P. Maestro, S. Marre, T. Delclos, C. Aymonier, P. Subra, L. Prat, C. Gourdon and E. Mignard, Lab Chip, 2011, 11, 779–787 RSC.
- B. J. Jin, Y. W. Kim, Y. Lee and J. Y. Yoo, J. Micromech. Microeng., 2010, 20, 350033 CrossRef.
- J. H. Xu, J. Tan, S. W. Li and G. S. Luo, Chem. Eng. J., 2008, 141, 242–249 CrossRef CAS.
- H. Song, D. L. Chen and R. F. Ismagilov, Angew. Chem., Int. Ed., 2006, 45, 7336–7356 CrossRef CAS.
- J. Jovanovic, E. V. Rebrov, T. A. Nijhuis, V. Hessel and J. C. Schouten, Ind. Eng. Chem. Res., 2010, 49, 2681–2687 CrossRef CAS.
- B. Zheng, J. D. Tice, L. S. Roach and R. F. Ismagilov, Angew. Chem., Int. Ed., 2004, 43, 2508–2511 CrossRef CAS.
- K. Wang, Y. C. Lu, J. H. Xu and G. S. Luo, Langmuir, 2009, 25, 2153–2158 CrossRef CAS.
- Y. J. Song, J. Hormes and C. Kumar, Small, 2008, 4, 698–711 CrossRef CAS.
- C. A. Serra and Z. Q. Chang, Chem. Eng. Technol., 2008, 31, 1099–1115 CrossRef CAS.
- C. N. Baroud, F. Gallaire and R. Dangla, Lab Chip, 2010, 10, 2032–2045 RSC.
- K. Wang, Y. C. Lu, J. H. Xu and G. S. Luo, Microfluid. Nanofluid., 2011, 10, 1087–1095 CrossRef CAS.
- I. Kobayashi, T. Takano, R. Maeda, Y. Wada, K. Uemura and M. Nakajima, Microfluid. Nanofluid., 2008, 4, 167–177 CrossRef.
- A. Gunther and K. F. Jensen, Lab Chip, 2006, 6, 1487–1503 RSC.
- A. S. Utada, A. Fernandez-Nieves, H. A. Stone and D. A. Weitz, Phys. Rev. Lett., 2007, 99, 94502 CrossRef.
- G. F. Christopher, N. N. Noharuddin, J. A. Taylor and S. L. Anna, Phys. Rev. E, 2008, 78, 036317 CrossRef.
- P. Garstecki, M. J. Fuerstman, H. A. Stone and G. M. Whitesides, Lab Chip, 2006, 6, 437–446 RSC.
- L. L. Shui, A. van den Berg and J. Eijkel, Microfluid. Nanofluid., 2011, 11, 87–92 CrossRef.
- W. Lee, L. M. Walker and S. L. Anna, Macromol. Mater. Eng., 2011, 296, 203–213 CrossRef CAS.
- C. Y. Lee, Y. H. Lin and G. B. Lee, Microfluid. Nanofluid., 2009, 6, 599–610 CrossRef CAS.
- M. Steegmans, K. Schroen and R. M. Boom, Langmuir, 2009, 25, 3396–3401 CrossRef CAS.
- S. L. Anna and H. C. Mayer, Phys. Fluids, 2006, 18, 121512 CrossRef.
- E. Castro-Hernandez, V. Gundabala, A. Fernandez-Nieves and J. M. Gordillo, New J. Phys., 2009, 11, 075021 CrossRef.
- T. Cubaud and T. G. Mason, Phys. Fluids, 2008, 20, 053302 CrossRef.
- Z. H. Nie, M. S. Seo, S. Q. Xu, P. C. Lewis, M. Mok, E. Kumacheva, G. M. Whitesides, P. Garstecki and H. A. Stone, Microfluid. Nanofluid., 2008, 5, 585–594 CrossRef CAS.
- B. Steinhaus, A. Q. Shen and R. Sureshkumar, Phys. Fluids, 2007, 19, 073103 CrossRef.
- W. S. Lee, S. Jambovane, D. Kim and J. W. Hong, Microfluid. Nanofluid., 2009, 7, 431–438 CrossRef CAS.
- D. X. Hao, F. L. Gong, G. H. Hu, Y. J. Zhao, G. P. Lian, G. H. Ma and Z. G. Su, Ind. Eng. Chem. Res., 2008, 47, 6418–6425 CrossRef CAS.
- H. Kim, D. W. Luo, D. Link, D. A. Weitz, M. Marquez and Z. D. Cheng, Appl. Phys. Lett., 2007, 91, 133106 CrossRef.
- S. Y. Park, T. H. Wu, Y. Chen, M. A. Teitell and P. Y. Chiou, Lab Chip, 2011, 11, 1010–1012 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary figures. See DOI: 10.1039/c2lc40159b. Some pictures of the experimental system. (a) The whole system. (b) The temperature constant air bath and the PID controller. (c) The air bath on the microscope plate. (d) The mini-evaporator with a 50 mL flask as reference. (e) The cooling unit. (f) The monitoring unit. |
| This journal is © The Royal Society of Chemistry 2013 |
