Battery and solid oxide fuel cell materials
Emma
Kendrick
*ab and
Peter R.
Slater
b
aSHARP Laboratories Europe Edmund Halley Road, Oxford, Oxfordshire, UK. E-mail: emma.kendrick@sharp.co.uk; Fax: +44 (0)1865 747717; Tel: +44 (0)1865 747711
bSchool of Chemistry, The University of Birmingham, Edgbaston, Birmingham B15 2TT, UK
First published on 14th May 2013
Abstract
This article reviews the literature reported during 2012 on battery and solid oxide fuel cell materials. The review focuses in particular on anode-, cathode- and electrolyte-materials for metal ion batteries such as lithium and sodium, and on oxide- and proton-conducting electrolytes, as well as anode- and cathode-materials used in solid oxide fuel cell applications.
Highlights
In the battery material field, recent work has focused upon the stabilisation of lithium excess materials, and other highlights include the investigation of an iron-containing MOF as a lithium ion cathode material. In the solid oxide fuel cell materials field, recent work has shown the successful accommodation of oxyanions (borate, silicate, phosphate) into perovskite electrode materials leading to some interesting improvements in properties. Other highlights include the use of microscopy to determine local deviations in oxygen content in perovskite systems.1. Battery materials
1.1 Lithium ion batteries
Capsoni et al.1 have reviewed the recent advances in lithium air batteries whilst Sun et al.2 have reported a room temperature sodium–air battery. The room temperature sodium air battery contained carbon thin film electrodes and exhibited 1884 mA h g−1 and 3600 mA h g−1 at 0.1 C and C/60 respectively with an average discharge voltage of 2.3 V vs. Na. Other areas of research have focused upon the specific components of a lithium ion battery and these are discussed in more detail below.High voltage materials. Kim et al.3 have synthesised LiNi0.8Co0.1Mn0.1O2 (NCM811) using alkali chlorides as a flux. This method produced segregated primary particles with well developed facet planes. The highly crystalline materials were shown to exhibit less gas emissions on high temperature storage when in a charged state and higher volumetric densities. With respect to the high voltage lithium excess materials, several methods to stabilise the cathodes have been performed and many studies have been undertaken to obtain a greater degree of understanding about the change in the material during first charge. In this respect a material with the formula Li[Li0.2Mn0.54Ni0.13Co0.13]O2 has been studied; the cathode was synthesised via a liquid phase route using polyvinylpyrrolidone as a carbon source. XPS and X-ray diffraction measurements indicated that there is no change in the oxidation state of the transition metals after surface treatment. Specific capacities of 293.2 mA h g−1 and 191.6 mA h g−1 were observed at 0.1 C and C rate in the voltage range 2.0–4.8 V vs. Li, with a capacity retention rate of ∼86% after 70 cycles at 1 C rate.4 The electrochemical properties can be attributed to the carbon surface coating, which is reported to minimise particle aggregation and cell polarisation, and hence improve the electronic conductivity. Oxide ion vacancies are promoted and a thick SEI layer is prevented. There has been much work this year on incorporating graphene into electrodes and Jiang et al.5 have synthesised a graphene and lithium excess composite material, (Li2MnO3)·LiMO2, M = Mn, Ni, Co, (LMNCO). Graphene coating reduced the overpotential of the cathode, and reduced the cell resistance, in particular the charge transfer resistance. Yu et al.6 have developed a 2-step molten salt process to synthesise a range of composites of formula xLi2MnO3·(1 − x)LiMn1/3Ni1/3Co1/3O2 (x = 0.1–0.4). The materials produced by this method show a particle size distribution ranging from 350 to 450 nm. The composites possess several transition metal oxidation states such as Mn4+, Ni2+, and Co3+ which are stabilised by lattice occupation of Li+ in both transition-metal layers and the lithium layer of LiMn1/3Ni1/3Co1/3O2. The composite where x = 0.3 exhibits a discharge capacity of 120 mA h g−1 at a high current density of 500 mA g−1 and a capacity retention of 64% after 20 cycles. At elevated temperatures the discharge capacity increases to 140.4 mA h g−1 at a high current density of 500 mA g−1, while average capacity decay rate becomes very small at 0.76%. Yu et al.7 have also investigated the materials using synchrotron X-ray powder diffraction. The activation mechanism of the Li2MnO3 phase is reported and the cycle stability elucidated based upon differential capacity and Raman spectroscopy studies. The reaction pathways are tracked via a compositional phase diagram of four components, Li2MnO3, LiMn0.42Ni0.42Co0.16O2, MO2 (M = Mn1−α−βNiαCoβ; 0 ≤ α ≤ 5/12, 0 ≤ β ≤ 1/6) and LiMnO2; this is reported to be an important tool for understanding and utilizing this ‘composite’ material. Zhang et al.8 have synthesised Li(Li0.17Ni0.25Mn0.58)O2 by a combination of co-precipitation and solid-state reaction. Surface nitridation was performed by heating the compound at 400 °C in an ammonia atmosphere. Nitrogen is reported at a trace level on the surface of the layered material, Li(Li0.17Ni0.25Mn0.58)O2 after nitridation. Compared to the as prepared material, the ammonia treated sample exhibited higher-rate capability and cycle stabilities. In situ X-ray diffraction and electrochemical analysis of these lithium excess materials show that there is strong anisotropy, as demonstrated in the shifts of the lattice parameters during the first cycle, and that there is dynamically changing strain in the first cycle due to lithium and transition metal migrations.9 Singh et al.10 have studied Li1.2Ni0.175Co0.1Mn0.52O2 by a range of analysis techniques. Large highly dense agglomerates of 5 μm in size are formed and X-ray diffraction studies show cation ordering in the transition metal layers. When charged up to 4.5 V vs. lithium the impedance of the cell and lithium cathode material decreased, above this voltage the impedance increased indicating a change in the lithium extraction mechanism of the material. From Raman spectroscopy it was observed that lithium extraction occurs from the transition metal layers and the lithium layers in the voltage range of 4.1–4.4 V vs. Li. Oxygen removal occurs in the voltage range of 4.55–4.6 V vs. Li, which is followed by transition metal migration to the vacant sites.
There is much work on synthesising and stabilising the high voltage spinel LiNi0.5Mn1.5O4 (LNMO), and in this respect Dong et al. have investigated the effect of particle size and crystallinity of LNMO.11 LNMO was synthesised by a solution evaporation process, with sintering at several increasing temperatures. As the sintering temperature was increased the size of the particles also increased and the crystallinity was enhanced. The samples prepared at 800 °C exhibited the best cycle reversibility, highest discharge capacity and optimal capacity retention. The stability of LNMO can also be improved by surface coating and Cho et al.12 have modified the surface by using a polyimide gel electrolyte. A continuous 10 nm coating was applied using a thermal imidization. The coating of the layered material produced a cathode which exhibited good ionic conductivity and also prevented unwanted side reactions at high voltages. The coated cathode was tested in a lithium metal cell at 55 °C, and the manganese dissolution, cell impedance and chemical composition on the LNMO surface were quantitatively analysed, showing an improvement over non coated high voltage spinels. Zhao et al.13 have coated LMNO with La0.7Sr0.3MnO3. The coating was made by mixing the LMNO in a sol–gel La0.7Sr0.3MnO3 matrix with subsequent high-temperature calcinations. The materials were tested in a lithium metal cell at elevated temperatures. When compared to non-coated LMNO, the coated material had much lower surface and charge transfer resistances with higher lithium diffusion rates. Electrochemical reversibility is improved, as is the stability at high temperatures. Prabakar et al.14 have investigated tungsten doping in LiWxNi0.5Mn1.5−xO4 (x = 0.00–0.10) which was synthesised using a sol–gel method. Tungsten was introduced into the reaction as W4+ but this was converted to W6+ in the final structure. At x = 0.005 W-doping enhances the electrochemical activity of the cathodes, with greater discharge capacities and less capacity fading reported than in the non doped material along with larger 4 V plateaus due to Mn3+/4+ being observed. An increase in Li–O bond length and decrease in transition metal bond length was observed in the x = 0.005 material. However increasing levels of tungsten caused lower electronic conductivities, and increased metal oxygen distances, resulting in poorer electrochemical performances.
Mg-doping in Li2MnSiO4 produces materials, which have the three-dimensional framework, monoclinic P21/n, structure (Fig. 1).21 Magnesium doping lowers the temperature of formation for the monoclinic silicate structure to 700 °C from 900 °C for the undoped material. In this work Li2MgxMn1−xSiO4 (x = 0.4 and 0.5) has been synthesised by the solid state route under argon at 700 °C. Poor electrochemical properties are still observed in Li2MgxMn1−xSiO4 due to its poor electronic properties and the large particle size of the solid-state synthesised products.
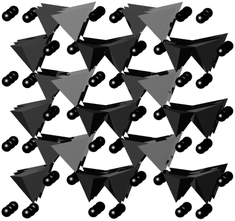 | ||
| Fig. 1 Atomistic structure of monoclinic Li2MgSiO4, light grey tetrahedra at Mg–O4, dark grey tetrahedra are SiO4, and black spheres are lithium. | ||
Kawase and Yoshitake22 have synthesised Li2MnSiO4 using a novel synthesis method involving mesoporous carbons. Highly dispersed Li2MnSiO4 was obtained in the carbon mesopores of CMK-3 and CMK-8 with high reversible capacities. Discharge capacities of 300 mA h g−1 were observed for Li2MnSiO4@CMK-3, which is close to the theoretical capacity. However volumetric energy densities are low due to the relatively low level of loadings in the highly porous carbon. Li2MnSiO4 has also been synthesised via a supercritical solvothermal method.23 Impurity free nanoparticles (15–20 nm) can be synthesised at 300 °C with very short reaction times of 5 min. After coating with conductive polymer, a discharge capacity of 313 mA h g−1 is observed. Longo et al.24 have studied silicate materials using density-functional theory (DFT) calculations. The olivine type structures and the tetragonal Pmn21 Li2MSiO4 (M = Mn, Fe, Co, and Ni) have been investigated. The calculations show the influence of exchange and correlation potentials used to compute the redox potentials, electronic band gaps and other properties of these materials.
Kohl et al.34 have used a sol–gel route to synthesise lithium transition metal fluorides of formula Li3MF6 and Li2MF5. Metal acetyl acetonates were used as precursors and the fluorides synthesised using a fluorolytic type process. 6Li and 7Li magic angle spinning (MAS) nuclear magnetic resonance (NMR) spectroscopy was used to study the local lithium ion environments and the results correlated well with the respective orthorhombic Li3VF6 and monoclinic Li2MnF5 structures and their structural lithium sites. Initial quantum chemical calculations were performed and high delithiation energies were reported, making them potentially suitable as high-voltage battery cathode materials. A novel metal organic phosphate has been reported as a lithium ion cathode material.35 The material K2.5[(VO)2(HPO4)1.5(PO4)0.5(C2O4)] has been investigated as a hybrid cathode material. The structure possesses 2-D pathways in the ab-plane and cavities along the c axis for the migration of the alkali ions. A schematic of the structure can be seen in Fig. 2.
![Atomistic schematic for the hydrated hybrid cathode material K2.5[(VO)2(HPO4)1.5(PO4)0.5(C2O4)]·4.5H2O showing the channels of water, with the layers of vanadium (black spheres), carbonates (white spheres) and phosphates and hydrogen phosphates (dark grey tetrahedra).](/image/article/2013/IC/c3ic90009f/c3ic90009f-f2.gif) | ||
| Fig. 2 Atomistic schematic for the hydrated hybrid cathode material K2.5[(VO)2(HPO4)1.5(PO4)0.5(C2O4)]·4.5H2O showing the channels of water, with the layers of vanadium (black spheres), carbonates (white spheres) and phosphates and hydrogen phosphates (dark grey tetrahedra). | ||
Graphene has been used extensively this year as an additive to known lithium ion anode materials to form composites with improved performance properties compared to composites without. In this respect Bhaskar et al.37 have investigated a MoO2–graphene composite synthesised via a one pot in situ low temperature solution phase reduction method. The graphene layers trap the MoO2 nanoparticles, with the presence of oxygen functionalities in the graphene allowing intimate contact between the oxide nanoparticles and the graphene. An initial discharge of 770 mA h g−1 was observed with a reversible capacity of 550 mA h g−1 after 1000 cycles. Cheekati et al.38 have investigated graphene nanosheets (GNS) and MnOx nanoparticle composites. It was reported that composites with 44% GNS and high MnO content delivered high reversible capacities, good cycling stability and good rate capabilities. Du et al.39 have reported the performance of Fe2O3–nitrogen-doped graphene composites as anode materials. The composites were synthesised by a one-step hydrothermal method. 100–200 nm size particles of Fe2O3 were deposited onto nitrogen-doped graphene. Reversible capacities of 1012 mA h g−1 were reported after 100 cycles with much improved rate capabilities. Capacities of 800 mA h g−1 were observed at a rate of 800 mA g−1. TiP2O7–graphene composites (10% graphene) have also been reported.40 These materials were synthesised using a co-precipitation synthesis route. At high rates the composite exhibited capacities of 98.4 mA h g−1 which is much higher than that of pure TiP2O7 (0.56 mA h g−1).
A TiO2-graphene composite was also investigated.41 The composite was synthesised by a gas/liquid interface reaction, 10 nm sized particles of TiO2 were deposited onto the graphene sheets. Electrochemical performance was investigated using a lithium metal anode, and the composites were cycled between 0.01 and 3 V vs. Li. High specific capacities of 499 mA h g−1 were observed with excellent rate capabilities. At high current densities of 3000 mA g−1, capacities of 150 mA h g−1 were observed.
Several spinel type materials have been investigated as lithium ion anode materials. Courtel et al.45 have synthesised ZnMn2O4 nanoparticles using a hydrothermal synthesis process. ZnMn2O4 was crystallised in a tetragonal spinel structure with a particle size of 5 nm. When cycled in a lithium metal cell, using lithium carboxymethylcellulose as a binder, a capacity of 430 mA h g−1 was observed after 100 cycles at C/10. Hu et al.46 have synthesised a MnxCo3−xO4 spinel type porous nanocube. The nano cubes were synthesised by a pyrolysis induced transformation of a Prussian blue analogue Mn3[Co(CN)]2·nH2O nanocubes. The electrochemical properties were tested in a lithium metal cell and high specific discharge capacities and good rate capabilities were observed. A nano structured CuFe2O4 has also been investigated.47 This material was synthesised by a polymer-pyrolysis method. Materials made at 700 °C exhibited the best cycling performance with a specific capacity of 552 mA h g−1 being observed after 100 cycles. Yoon et al.48 have investigated nitrided MoO2 synthesised by a hydrothermal process followed by post nitridation in ammonia. Molybdenum nitride (γ-Mo2N and δ-MoN) and molybdenum oxynitride (MoOxNy) are observed and specific capacities of 420 mA h g−1 are reported after 100 cycles. The properties of several MoSi2 and CrSi2 silicides have also been investigated. The two materials were made using high impact ball milling of the corresponding metals. MoSi2 was formed after 20 hours, however CrSi2 required over 100 h. Specific capacities of 340 mA h g−1 and 167 mA h g−1 were observed at C/12 and C/2 rates respectively. Cui et al.49 have investigated the electrochemical properties of LiSn2(PO4)3 NASICON type structured material. The material was synthesised using a solid state method at 900 °C. A specific capacity of 320 mA h g−1 was observed after 50 cycles.
Lee et al.50 have synthesised modified Co3O4 composites via organometallic complexation of cobalt carbonyl with microporous organic networks (MON's). These composites show improved stabilities as anode materials compared to the unmodified Co3O4. The materials were tested in a lithium metal cell with discharge from 1 mV to 3 V at a rate of 50 A g−1. Nanoparticles prepared by the same method but with no MON modifications exhibited a sharp decrease in capacity after 30 cycles to 90 mA h g−1, whereas the MON modified samples MC-30 and MC-60 exhibited higher discharge capacities of 420 and 640 mA h g−1 respectively. Stable cycling, improved rate capabilities and high Coulombic efficiencies were also observed. The enhanced stability of these composites is reported to be due to the MON blocking the formation of aggregates and hence increasing the discharge stability. The electrochemical lithium insertion properties of Ti2Nb10O29 have been investigated. The material has a ReO3 type structure.51 Electrochemical properties for lithium insertion were measured in the voltage region 1.0–2.5 V and it was observed that up to 15 lithium could be inserted per formula unit, leading to a corresponding specific capacity of 270 mA h g−1 upon 1st discharge. From in situ XRD techniques, Ti2Nb10O29 was observed to form a solid solution upon lithium insertion followed by a phase separation. Zeng et al.52 have synthesised single crystal TiOF2 nanotubes by a one-step solvothermal method, with the dimensions in the region of 2–3 μm by 200–300 nm, and the walls around 40–60 nm thick. TiF4 and oleic acid in octadecane were used to form the nano tubes. The TiOF2 nanotubes possess a lower voltage for lithium intercalation than TiO2 and exhibit higher specific capacities, stable cyclabilities and good rate capabilities. Xu et al.53 have investigated the anodic properties of Li2FeSiO4 nanoparticles. The materials were first made by a sol–gel method, leading to particles of 20–40 nm in size being produced with a thin film of amorphous carbon. An initial discharge capacity of 880 mA h g−1 was observed upon first discharge, and after 173 cycles, 449 mA h g−1 was observed at a 100 mA g−1 rate of charge and discharge.
Iron and cobalt fluorides have been investigated as conversion anode materials for lithium ion batteries. Li et al.54 have synthesised FeF3 nanowires, and these wires form continuous networks of iron upon reduction. Initial discharge capacities of 543 mA h g−1 were observed, with a reduction to 223 mA h g−1 after 50 cycles at room temperature. The loss of active material during cycling, caused by an incomplete conversion process, was the reason for the capacity loss during cycling. Liu et al.55 have synthesised and tested a cobalt doped FeF3 material, Fe1−xCoxF3 (x = 0, 0.03, 0.05, 0.07), made by a liquid phase method. Composites of the fluorides and acetylene black were made for testing in an electrochemical cell. Cobalt doping was observed to greatly improve the electrochemical cyclability, however much lower discharge capacities were observed. Discharge capacities of 136 mA h g−1 were observed at C rate with 92% capacity retention after 100 cycles.
Kokal et al.61 have investigated a Li6BaLa2Ta2O12 (LLBTO) sample, which was prepared by a modified sol–gel Pechini method. The precursors were annealed at temperatures between 923 and 1123 K for 6 hours in air. LLBTO exhibited a total Li-ion conductivity of 1.69 × 10−5 S cm−1 at 298 K with an activation energy of 0.40 eV. NASICON-type structures are also well known solid electrolyte materials and LATP (Li1+x+yAlxTi2−ySiyP3O12) has been previously investigated as a solid electrolyte material. Ding et al.62 have investigated LATP as a material for aqueous batteries and hence investigated the H+ diffusion properties. Modelling and experimental studies show that H+ ions can be adsorbed onto the glass surface and bulk H+ diffusion is not observed due to the very high diffusion barrier (3.21 eV). Surface corrosion was observed in strongly alkaline solutions and therefore alternative electrolytes need to be developed for long term operation in Li–air batteries. A sodium analogue (Na1+xZr2SixP3−xO12) has been investigated by Yadav et al.63 The ionic conductivity of the material was improved by varying the composition of the material. Results indicate that conductivity was enhanced for materials where x = 2.05, the reason was attributed to changes in the orientations of the tetrahedra which lead to a change in the bottle neck sizes. Ohta et al.64 have investigated the electrochemical performance of an all solid state battery comprised of a LiCoO2 cathode, Li6.75La3Zr1.75Nb0.25O12 (LLZONb; solid electrolyte), and a lithium metal anode. A favourable charge and discharge behaviour was observed with no other phase reaction or exfoliation observed at the LCO and LLZONb interface after 100 cycles. The interfacial resistance was comparable to that of lithium ion batteries with a liquid electrolyte and the activation energy of the interfacial resistance was lower.
1.2 Sodium ion batteries
Materials for sodium ion batteries are gathering more interest due to their potential as a lower cost alternative to lithium ion. Ellis et al.65 have reviewed the challenges for sodium ion batteries and have reviewed the emerging sodium ion technologies, which show promise as a replacement for lithium ion technologies. Palomares et al.66 have also reviewed the recent advances and challenges of sodium ion batteries for low cost energy storage systems. Whitacre et al.67 have discussed the economics of large format stationary energy storage devices which use an aqueous sodium ion technology. In this technology asymmetric devices which are composed of a MnO2 positive electrode and an activated carbon negative electrode were used. Results indicated that the use of this type of hybrid device is only suitable when energy density is not a concern, but the technology has been demonstrated successfully up to large format >30 W h devices.Several phosphate materials have been investigated as sodium ion cathode materials and in this respect Barpanda et al.68 have synthesised a novel iron based 3 V pyrophosphate cathode material, Na2FeP2O7. This triclinic structure has 3-dimensional channels and has been synthesised by a solid state and a solution-combustion synthesis route. A reversible capacity of 82 mA h g−1 was observed at 3 V vs. Na/Na+. Jian et al.69 have synthesised a carbon coated cathode Na3V2(PO4)3 using a one step solid state reaction. Two voltage plateaus were observed at 1.63 V and 3.4 V vs. Na. When cycled at the higher voltage, initial charge and discharge capacities of 98.6 mA h g−1 and 93 mA h g−1 are observed respectively, with 99% capacity retention after 10 cycles. When tested as an anode material a specific capacity of 59 mA h g−1 was observed after 50 cycles. Kang et al.70 have synthesised a Na3V2(PO4)3–C cathode by a polyol-assisted method. A discharge capacity of 235 mA h g−1 was observed, which corresponds to an extraction of 4 Na per formula. 56% of theoretical capacity was observed at 2.67 C rate. NaMn1/2Co1/3Ni1/3PO4 has been investigated for use as a water based cathode material in Na–ion batteries. This maricite-type material is isostructural with the olivine based structure although the M1 and M2 sites have reversed occupancies. Initial results indicate that the sodium analogue is fully reversible for sodium intercalation and deintercalaction in an aqueous NaOH electrolyte. Oh et al.71 have investigated NaFePO4 obtained by electrochemical sodium exchange for lithium in LiFePO4. X-ray diffraction indicated that the structure is retained during cycling over 50 cycles. The material can be cycled with an operating voltage of 2.7 V vs. Na and has a stable capacity of 125 mA h g−1. A3V2(PO4)3 (A = Na, Li) has been synthesised using the citric acid assisted sol–gel method. When tested vs. Li or Na anodes respectively, both materials gave capacities of 90 mA h g−1. The local environment was probed by in situ X-ray absorption spectroscopy and it was observed that there was a small relaxation in the local structure during alkali metal extraction, however both structures were rigid with no significant changes observed. Sun et al.72 have synthesised the NaFePO4 maricite phases. When tested vs. Li with a lithium ion electrolyte an increase in capacity was observed over several cycles with consistently low resistance.
Layered sodium transition metal oxides are also of interest and carbon-coated NaCrO2 has been synthesised and investigated as a cathode material. The discharge capacity after 40 cycles remains at 110 mA h g−1. Kim et al.73 have investigated a NaNa1/3Fe1/3Mn1/3O2 cathode using an organic ester carbonate and sodium salt electrolyte. The cathode was synthesised using a solid state reaction at 850 °C with slow cooling. An average voltage of 2.75 V vs. Na was observed with a capacity of 100 mA h g−1 over 150 cycles. The cathode was analysed during cycling using X-ray diffraction techniques and it was observed that the layered structure was maintained for the material Na1−yMO2 (0 ≤ y ≤ 0.46). Hosono et al.74 have investigated high power sodium ion batteries using a nanostructured Na0.44MnO2 single crystal nanowire. The manganese oxide nanowire was made by the hydrothermal method and exhibited good cyclability and high rate capability. Rasul et al.75 have investigated layered birnessite and tunnel structure hollandite manganese oxides as high capacity cathodes for magnesium ion batteries. These materials were studied in the voltage range −1.8–1.0 V vs. Ag/Ag+. Highest discharge capacities of 109 mA h g−1 were observed for birnessite, whilst hollondite exhibited 475 mA h g−1 at 60 °C. Large capacity losses were observed upon cycling due to magnesium trapping in the structures.
Kitajou et al.76 have investigated the electrochemical properties of the perovskite-type NaFeF3. An initial discharge capacity of 197 mA h g−1 was observed between 1.5 V and 4.5 V vs. Na. A reversible Fe2+/Fe3+ redox reaction was confirmed by XPS. Shakoor et al.77 have synthesised Na3FeF6 by mechanochemical synthesis. NaF and FeF3 precursors were ball milled under argon and a monoclinic structure was formed which is stable up to temperatures of 500 °C. When tested in an electrochemical cell vs. a lithium or sodium anode, reversible capacities of 100 and 200 mA h g−1 were respectively observed at room temperature. Lee et al.78 have investigated a sodium zinc hexacyanoferrate, Na2Zn3[Fe(CN)6]2·xH2O, with a well defined open framework structure, as a cathode for sodium ion batteries. A voltage of 3.5 V vs. Na was observed with a reversible capacity of 56.4 mA h g−1 and good cycle life. Lu et al.79 have discussed the use of Prussian blue and its analogues for cathodes in sodium ion batteries. The compounds (with Fe, Mn, Ni, Cu, Co and Zn) have been synthesized at room temperature. Reversible sodium insertion was observed for KFe2(CN)6 in a carbonate electrolyte, and capacities of 100 mA h g−1 were reported with no capacity fade in 30 cycles.
In terms of anode materials for sodium ion batteries, Komaba et al.80 have investigated the use of tin as an anode for sodium ion batteries. Reversible capacities were observed for Sn vs. Na with 1M NaClO4 in PC electrolyte. Improvements in the reversible capacities were observed with polyacrylate binders and fluoroethylene carbonate addition to the electrolyte. In terms of sodium ion solid electrolytes Fergus81 has reviewed the ion transport properties in sodium ion conducting solid electrolytes. Reynaud et al.82 have reported the Na-based magnesium, copper and zinc fluorosulphates, of formula NaMSO4F which crystallise with the maxwellite (tavorite-like) frame work, similar to the iron analogies. Conductivities of 10−7 S cm−1 and 10−11 S cm−1 were reported depending on the synthesis process with no reversible electrochemical activity being observed vs. Na.
2. Solid oxide fuel cells
There have been a number of useful review articles published in 2012 which provide a background to some fundamental aspects of solid oxide fuel cell (SOFC) materials. In particular, attention is drawn to reviews by Aguadero et al. on materials for intermediate temperature solid oxide fuel cells and electrolysers,83 Laguna-Bercero on advances in high temperature electrolysers84 and by Fabbri et al. on solid oxide fuel cells utilising proton conducting electrolytes.852.1 Electrolytes
Fluorite-type materials, such as rare earth doped ZrO2, CeO2 continue to be the most favoured electrolyte systems for solid oxide fuel cell applications. Prior studies of these fluorite materials have shown that the conductivity decreases in heavily doped systems, attributed to the fact that not all the oxygen ion vacancy defects are mobile. Through combining impedance and 89Y NMR studies, Chen et al. have investigated the effective concentration of mobile vacancies in heavily doped (59 cat% Y) YSZ,86 showing that it is significantly lower than the nominal vacancy concentration. The activation energy for migration was shown to be significantly higher than in 17 cat% Y samples, indicating significant defect interactions in the heavily doped systems. In related work Burbano et al. have investigated oxygen vacancy ordering in yttria doped CeO2 through a combination of neutron diffraction, impedance spectroscopy measurements and computer modelling.87 The authors suggest that there is a significant influence on the conductivity from oxide ion vacancy–vacancy interactions, with the formation of vacancy–vacancy pairs along the 〈111〉 direction, hence accounting for the lowering in conductivity for high dopant levels. In related work, Miller et al. have utilised modelling studies to investigate oxide ion ordering in fluorite systems.88 Their work suggests that, in the vicinity of oxide ion vacancies, there can be displacement of neighbouring oxide ions into interstitial sites. This is particularly the case for Sc-doped systems and the authors suggest that this may explain the reduction in conductivity in scandia doped fluorite electrolytes over time.The addition of TiO2 has been reported to improve the sintering and to lower the grain boundary resistance of Gd0.2Ce0.8O1.9.89 The optimum (0.8 mol%) Ti content was shown to be just above the solubility limit of Ti (0.6 mol%) in the structure, suggesting that the presence of some Ti at the grain boundary provides a beneficial effect on the grain boundary conductivity.
There has been a lot of interest in potential low temperature proton conductivity in nanocrystalline fluorite-type oxide ion conductors following recent results of significant room temperature conductivity in such systems in wet atmospheres. Detailed work by Tande et al. has, however, shown that the room temperature enhancements seen in such samples is simply due to surface conduction, with bulk transport being shown to be negligible.90
In terms of other fluorite work, Huang et al. have examined composites of Y0.2Ce0.8O1.9 and BaZr0.1Ce0.7Y0.2O2.9 sintered at 1400 °C.91 While lower conductivities than the individual materials were observed in dry air, enhanced conductivities were observed in wet H2, up to 0.033 S cm−1 at 500 °C.
There has also been continued interest in low temperature solid oxide fuel cells, making use of Bi-based fluorite-type electrolytes, which show the highest reported oxide ion conductivities, but are incompatible with high temperature (≥500 °C) operation due to reduction of Bi. Lee et al. have shown that the electrolyte with composition Dy0.08W0.04Bi0.88O1.56 has very high oxide ion conductivity, and the authors have investigated its' application in low temperature SOFCs.92 The authors utilised a functionally graded bilayer Bi2O3/CeO2 based electrolyte to overcome the instability issues in reducing atmospheres, and have demonstrated good performance for cathode systems containing Bi2Ru2O7 in conjunction with such electrolytes. Use of this bilayer electrolyte allowed the authors to produce fuel cells capable of high power densities (2 W cm−2 at 650 °C).
Apatite-type electrolytes have continued to attract significant interest, with the oxide ion conductivity in these systems being mediated by interstitial oxide ion- rather than vacancy- defects. Further studies of c axis orientated apatite materials have been reported by Fukuda et al.93 Systems containing oxygen excess were prepared by a diffusion reaction at 1600 °C for 50 hours between La2SiO5:La2Si2O7:La2SiO5. An oxygen excess sample with composition La9.5Si6O26.25 showed higher conductivity than the oxygen stoichiometric system La9.33Si6O26 due to the higher interstitial oxide ion defect concentration. The same authors have also examined the effect of partial substitution of Si by Ge, by using a La2Si2O7:La2Si0.87Ge0.13O5:La2Si2O7 reaction couple.94 The resulting La9.33(Si0.87Ge0.13)6O26 showed higher c axis conductivity than La9.33Si6O26.
In terms of providing further understanding of the conduction mechanisms in these apatite systems, Matsunaga and Toyoura have performed first principle calculations on the apatite system La9.33+xSi6O26+3x/2.95 The most favourable interstitial oxide ion site was reported to lie close to the channel oxide ions, with conduction along the c direction predicted via an interstitialcy mechanism. The authors also reported a pathway for conduction perpendicular to the c axis involving the SiO4 units.
Further information on the conduction mechanism has been provided by 17O NMR studies on the apatite system La9.33Si6O26.96 The samples were 17O enriched through heat treatment in a Pt cell at temperatures between 100 and 1000 °C in 17O enriched water vapour. Peaks assigned to the silicate oxygens were found at chemical shifts ≈200 ppm, while a small peak attributed to the channel oxygen was found at ≈600 ppm. From high temperature static NMR measurements, the authors concluded that the mobile oxide ions are those bonded to Si, in line with suggestions of the importance of the SiO4 units in the conduction process.
While research on apatite-type systems has principally focused on oxide ion conductivity, as outlined above, high proton conductivity (approaching 0.01 S cm−1 at 700 °C) has been reported in Y-doped hydroxyapatite by Wei and Yates showing the versatility of the apatite structure with regards to ionic conduction.97 The authors prepared dense aligned membranes, grown hydrothermally on a Pd substrate, and the conductivity was shown to be predominantly due to protons with an activation energy of 0.73 eV.
In terms of other Si/Ge containing systems, Singh and Goodenough have reported high oxide ion conductivity in Sr1−xKxSi1−yGeyO3−x/2.98 These systems contain (Si/Ge)3O9 units and the introduction of K leads to oxide ion vacancies and accompanying high oxide ion conductivity, with the highest value being reported for Sr0.8K0.2Si0.5Ge0.5O2.9 (0.01 S cm−1 at 625 °C).
Perovskite systems have also attracted significant interest as SOFC electrolytes, with growing work on the development of Ba(Zr/Ce)O3 based proton conducting systems.
Enhancements in the performance of the perovskite proton conducting system, BaCe0.9Y0.1O2.95 has been reported through Ni-doping by Caldes et al.99 The authors first prepared a Ni-doped phase, BaCe0.7Y0.1Ni0.2O3−x, which after reduction led to the precipitation of Ni nanoparticles. The reduced phase showed enhanced proton conductivity in H2, attributed to the effect of the Ni nanoparticles improving the incorporation of protons through hydrogen dissociation.
Proton conducting perovskite ceramics can also be exploited as hydrogen separation membranes and can been used to increase H2 yields in water–gas shift reactors. In this latter area, thin SrCe0.7Zr0.2Eu0.1O3−y membranes (on Ni-SrCe0.8Zr0.2O3−y supports) have been demonstrated as promising, with the introduction of Zr improving the stability of the membrane under the reactor conditions.100
The characteristics of the proton conductor BaYxSn1−xO3−x/2 (0.1 ≤ x ≤ 0.5) have been analysed by 119Sn, 89Y and 17O NMR and DFT studies.101 At low levels of Y doping (x ≤ 0.2) substitution was shown to be random, but with increasing Y content, cation ordering occurred such that for x = 0.5 a Y–O–Sn network was observed. The authors attribute the high proton conductivity in this material to this Y–O–Sn network which helps to prevent proton trapping that might be expected if Y–O–Y sites were present. The stability of this system has been examined by Wang et al.102 While high Y contents lead to the highest bulk proton conductivities, such samples were also shown to be the least stable, suggesting that in terms of applications a balance between these two features needs to be met.
One issue with rare earth doped BaZrO3 proton conductors is their generally poor grain boundary conductivity. The blocking nature of the grain boundaries has been attributed to space charge effects, and evidence in support of this has been provided by Shipour et al., where the authors showed a disappearance of this large grain boundary resistance in heavily reduced n-type BaYxZr1−xO3−y.103
Ti-doped Ba2In2O5 has continued to attract interest for its high proton conductivity. Co-doping with different rare earths (Gd, Dy, Ho, Er, Yb, Lu) has been examined by Jarry et al.104 The best performance was for Yb-doping, with a proton conductivity of 1.7 × 10−3 S cm−1 at 400 °C for BaIn0.6Ti0.2Yb0.2O2.6.
The related Sc-containing system, Ba2Sc2O5, has also attracted interest, and phosphate groups have been successfully incorporated into this material to give cubic perovskite phases, Ba2Sc2−xPxO5+x (x = 0.4, 0.5), showing high proton conductivities (up to 6 × 10−3 S cm−1 at 500 °C) in wet atmospheres, along with good CO2 stability.105 In addition, this work suggested that undoped Ba2Sc2O5 does not exist, and is only stabilised at temperatures below 1000 °C by the presence of carbonate to give a Sc-deficient perovskite phase, Ba2Sc2−xCxO5+x/2.
Other systems displaying proton conduction have also attracted attention. A detailed analysis of La2Ce2O7 has been performed by Besikiotis et al.106 Proton conductivity was shown to be the dominant conduction process below 450 °C, confirmed by H/D isotope measurements, with oxide ion conductivity dominating above this temperature. The total conductivity varied from ≈10−5 S cm−1 (at 400 °C) to ≈10−1 S cm−1 (at 1000 °C).
Lanthanum tungstate phases have also been attracting growing interest due to the observation of good mixed (H+ and electronic) conductivity in La28−yW4+yO54+δ. Amsif et al. have investigated Mo-doping (in place of W) in this system, with the results showing a change in cell symmetry from cubic to rhombohedral on increasing Mo doping.107 Good mixed conductivity under wet reducing conditions was observed for La28−y(W1−xMox)4+yO54+δ with x ≤ 0.4, while for higher Mo contents electronic conductivity dominated. The authors suggested applications for samples with x ≤ 0.4 as hydrogen separation membranes.
Further studies of metal phosphate systems for use as proton-conducting electrolytes below 500 °C have been reported. Huang et al. have prepared niobium phosphates (from Nb2O5 and H3PO4) with proton conductivities between 1.6 × 10−2–5.8 × 10−2 S cm−1 at 250 °C, depending on pH2O, with the work also reporting the existence of hydroxyl groups even after heat treatment to ≥500 °C.108 Mixed Fe- and Ta-phosphates have also been examined, with power densities as high as 0.34 W cm−2 reported for a fuel cell using a Fe0.4Ta0.5P2O7−x electrolyte.109
2.2 Cathode materials
Perovskite transition metal-containing systems continue to dominate the research into cathode materials for SOFCs, due to their high conductivities and catalytic activity for the oxygen reduction reaction. Cobalt-containing perovskites have attracted considerable interest due to their accompanying high oxide ion conductivity. In terms of fundamental studies, Kim et al. have performed detailed scanning transmission electron microscopy measurements to determine the local concentrations of oxide ion vacancies in perovskite cobaltate systems.110 Specifically, the authors elegantly demonstrate that by determining the local lattice parameter variations, the oxygen vacancy concentration can be effectively mapped.Ba0.5Sr0.5Co0.8Fe0.2O3−y and related phases have continued to attract considerable interest due to their excellent electronic and oxide ion conductivity. A number of studies, however, have shown that this phase undergoes a gradual change from a cubic to a hexagonal perovskite on heating at intermediate temperatures. Mueller et al. have investigated this degradation in more detail through microscopy studies and observed a further plate-like phase.111 This phase was shown to be deficient in Sr and enriched in Co, and was shown to form faster than the hexagonal phase. In terms of overcoming this degradation issue, doping of Ba1−xSrxCo0.8Fe0.2O3y with borate, and phosphate has been reported to help to stabilise the cubic perovskite phase.112 5% phosphate doping was shown to lead to the best performance, with the cubic phase stabilised and hence high electronic conductivity maintained even after annealing at intermediate temperatures. Ti-doping has also been examined to improve the long term stability of Ba0.5Sr0.5Co0.8Fe0.2O3−y in the presence of CO2.113 Up to 10% Ti substitution in place of Co/Fe was examined with the best stability shown for the highest Ti content. A fuel cell based on a BaZr0.4Ce0.4Y0.2O3−y electrolyte utilising a composite cathode of Ba0.5Sr0.5(Co0.8Fe0.2)0.9Ti0.1O3−y with the electrolyte yielded a maximum power density of 0.18 W cm−2 at 700 °C.
In terms of long term stability under operation, the compatibility of the fuel cell cathode materials in conjunction with the electrolyte is vital for reliable stable performance. In this area, Yung et al. have observed enhanced reactivity between Ba0.5Sr0.5Co0.8Fe0.2O3−y electrodes and ceria based electrolytes under cathodic polarisation leading to the formation of barium cerate.114 The reactivity was attributed to Ba segregation under the cathodic polarisation. Ni-doping (5 wt%) has been shown to improve the performance of Ba0.5Sr0.5Co0.8Fe0.2O3−y electrodes by acting as a grain growth inhibitor.115
SrCoO3−δ has also continued to attract interest, and Aguadero et al. have examined 5–10% Mo-doping in this phase.116 The Mo-doping was shown to convert the system from a hexagonal to a tetragonal perovskite, with an accompanying enhancement in the electronic conductivity. Good performance was observed under both cathodic and anodic conditions, and the authors suggest that this material may be suitable as a reversible oxygen electrode for cells capable of operating as both SOFCs and solid oxide electrolysers.
Traditionally the cobalt containing perovskites utilised in SOFC applications contain alkaline earths, so as to introduce the oxide ion vacancies to mediate the oxide ion conductivity. Somewhat surprisingly LaCoO3, without alkaline earth doping, has been reported to show excellent performance as a cathode in conjunction with proton-conducting perovskite electrolytes.117 The cathode was formed by infiltration of LaCoO3 into porous BaY0.1Ce0.2Zr0.7O3−y on top of a dense membrane of the same electrolyte. Cathode ASRs of 0.39 and 0.11 Ω cm2 were reported at 500 and 600 °C respectively. In related work, Samson et al. have examined the performance of different cathodes consisting of porous Gd0.1Ce0.9O1.95 onto which La0.6Sr0.4CoO3−y, LaCoO3, Co3O4 was infiltrated (using solutions containing the respective metal nitrates).118 While the best performance was for La0.6Sr0.4CoO3−y, good performance was also observed for the other two systems despite their apparent lack of oxygen nonstoichiometry.
La0.6Sr0.4Co0.8Fe0.2O3−y has also been proven to be an excellent electronic- and oxide-ion conductor, and strategies for improving the properties further have been targeted. Vert et al. have shown enhanced oxide ion diffusion in a Ba- and Pr-doped (La/Sr)Fe0.8Co0.2O3−y.119 Through isotopic tracer diffusion experiments in the temperature range 450–650 °C, the composition La0.2175Pr0.2175Ba0.145Sr0.4Fe0.8Co0.2O3−y was shown to display excellent oxide ion diffusion. Improvements in the performance of La0.6Sr0.4Co0.8Fe0.2O3−y have also been reported through coating with Sm-doped CeO2.120 An order of magnitude improvement in the surface exchange rate was observed, indicating the strongly beneficial effect of the doped CeO2 additions.
The perovskite LaSrMnCoO5+y has also been examined as a cathode.121 High conductivities were observed along with low polarisation resistances (0.048 Ω cm2 at 800 °C) in conjunction with a Sm0.2Ce0.8O1.9 electrolyte.
Cobalt-containing double perovskites have also been targeted as SOFC cathodes, and Li et al. have examined in detail the mixed Co2+/Co3+ double perovskites Ba2Sc0.2BixCo1.8−xO6−y (x = 0.1, 0.2).122 Neutron diffraction studies confirmed that these were indeed double perovskites, with Co in one B cation site, and Co, Bi (as Bi5+), Sc in the other B cation site. The material showed a large level of oxide ion vacancy defects (y ≈ 1.3 at room temperature), with no evidence for absorbed water.
Burriel et al. have examined oxygen diffusion and surface exchange characteristics in PrBaCo2O5+δ materials.123 The authors observed inhomogeneity in the 18O enrichment from grain to grain, which was attributed to anisotropy in the oxide ion diffusion and surface exchange, as predicted from prior computer modelling studies. Through a combination of experiments, this anisotropy was evaluated, with the results indicating that diffusion was faster for ab-plane orientated grains compared to c-axis orientated grains. In related work, Hu et al. have examined the mechanism of oxide ion diffusion in NdBaCo2O5+δ through a maximum entropy approach to the analysis of neutron diffraction data at high temperatures (900 °C), coupled with molecular dynamics studies.124 Both the experimental and modelling results showed an anisotropic conduction process, with a similar complex oxide ion diffusion pathway in the Nd–Co ab-plane, with conduction in the c direction being limited by the lack of oxide ion vacancies in the Ba-containing planes.
In terms of other cobalt-containing perovskites, the system La0.5Sr0.5Co0.5Ti0.5O3−y has been examined for use both as a cathode and an anode in SOFCs.125 The phase was shown to have a conductivity of 29 S cm−1 at 850 °C with good performance as a cathode in conjunction with a LaGaO3 based electrolyte. It was also shown that this phase could be used both as a cathode and an anode at 800 °C, with the resultant SOFC yielding a maximum power density of 0.11 W cm−2.
Rao et al. have investigated Co-doped BaZrO3 as an electrode for a reversible SOFC based on a proton conducting electrolyte.126 Co levels on the perovskite B cation site up to 50% were examined, with an increase in electronic conductivity with increasing Co-content. A polarisation resistance of 0.19 Ω cm2 was obtained for BaZr0.6Co0.4O3−y at 700 °C, which was lower than that observed for other electrode materials, such as Sm0.5Sr0.5CoO3−y.
Grimaud et al. have investigated the performance of a range of perovskite-related materials as potential cathode materials in conjunction with a perovskite proton conducting electrolyte.127 Of the materials examined, evidence for the presence of protons was observed for Ba0.5Sr0.5Co0.8Fe0.2O3−y, PrBaCo2O5+y and Pr2NiO4+y, with the largest degree of water uptake observed for Ba0.5Sr0.5Co0.8Fe0.2O3−y.
Potential cathodes have been examined for use with proton conducting La5.5WO11.25 by Solis et al.128 The authors examined a composite cathode with La0.8Sr0.2MnO3−y (LSM), which showed improved performance compared to LSM on its own, with the best performance for the cathode containing 40% La5.5WO11.25. Other cathode materials have also been investigated in conjunction with this electrolyte system, including La0.75Sr0.25Cr0.5Mn0.5O3−y and Ba0.5Sr0.5Co0.8Fe0.2O3−y.129 In this work reaction with the electrolyte was shown for Ba0.5Sr0.5Co0.8Fe0.2O3−y, while La0.75Sr0.25Cr0.5Mn0.5O3−y and La0.7Sr0.3MnO3−y were shown to be chemically stable. The best performance was shown for a composite of La0.7Sr0.3MnO3−y and the electrolyte.
Due to the generally high thermal expansion coefficients and reactivity of Co-containing systems, non-cobalt containing perovskites have also been widely researched. The effect of the rare earth size on the electrical properties of Ln0.8Sr0.2FeO3−y (Ln = La, Pr, Sm) has been investigated by Ren et al.130 The ionic conductivity was reported to be highest for Ln = Pr, while the electronic conductivity was highest for Ln = La. Overall there was, however, little difference between the cathode performance for the three systems.
GdBaNiFeO5+y has been reported to be a good cathode material for use in conjunction with a proton-conducting BaY0.2Zr0.1Ce0.7O3−y electrolyte by Yang et al.131 A low interfacial polarisation resistance (0.15 Ω cm2 at 700 °C) was reported, and a fuel cell with this cathode material gave high power densities (up to 0.456 W cm−2 at 700 °C).
Ni (up to 15%) doped La0.4Sr0.6FeO3−x has been examined as a cathode material in conjunction with a BaZr0.1Ce0.7Y0.2O3−y.132 The highest conductivity (160 S cm−1 at 400 °C) was observed for the 10% Ni doped sample. A cell with this cathode and a Ni–BaZr0.1Ce0.7Y0.2O3−y anode gave power densities as high as 0.4 W cm−2 at 700 °C. In related work, Ba0.5Sr0.5Fe0.9Ni0.1O3−y has been examined in conjunction with Sm0.2Ce0.8O1.9 as a cathode for use with the same electrolyte.133 A similar power density (0.36 W cm−2) was observed with this composite cathode. A related system containing Nb, Ba0.5Sr0.5Fe0.9Nb0.1O3−y has also been reported as a promising potential cathode material with a reported conductivity as high as 14 S cm−1 at 450 °C. This cathode deposited on a Sm0.2Ce0.8O1.9 electrolyte gave a low ASR (0.082 Ω cm2), and a full cell with this cathode gave high power densities (up to 0.745 W cm−2 at 700 °C).134
The related Ba0.5Sr0.5Fe0.9Mo0.1O3−y has been investigated as part of a composite cathode system with BaY0.2Zr0.1Ce0.7O3−y by Yang et al.135 Ba0.5Sr0.5Fe0.9Mo0.1O3−y was shown to have high conductivity (192 S cm−1 at 400 °C in air), and a fuel cell with this material, in a composite cathode in conjunction with an BaY0.2Zr0.1Ce0.7O3−y electrolyte, gave power densities as high as 0.42 W cm−2 at 700 °C.
In terms of novel doping strategies, the incorporation of borate and phosphate into perovskite manganates has been demonstrated.136 In the case of CaMnO3, such a doping strategy led to an increase in the electronic conductivity due to partial reduction of the Mn oxidation state and hence introduction of mixed valency, while for La1−xSrxMnO3−y there was a small decrease in electronic conductivity. Composites of these manganates with Gd0.1Ce0.9O1.95 (CGO) were analysed on CGO pellets. For both systems, there was a reduction in the area specific resistivity values for the borate/phosphate doped samples showing the beneficial effect of this doping strategy. Similar successful incorporation of low levels (up to 5%) of borate, silicate and phosphate has been reported for Sr0.9Y0.1CoO3−y and La0.6Sr0.4Co0.8Fe0.2O3−y.137 In all cases, the oxyanion doping was shown to lower the ASR values, while for Sr0.9Y0.1CoO3−y improvements in the CO2 stability were also observed.
Chen et al. have investigated the influence of segregation of Sr at the surface of fuel cell cathode materials using SrTi1−xFexO3−y as a model system.138 The results indicate that segregation of Sr on the surface is detrimental to the oxygen reduction reaction, confirmed by the fact that removing such surface layers through chemical etching leads to a significant improvement in the performance. In related work, Ren et al. have reported an enhancement in the performance of La1−xSrxMnO3−y/YSZ cathode materials through treatment with an alkali solution.139 The treatment was shown to remove Sr species that had segregated to the surface.
K2NiF4-type rare earth nickelates have also continued to attract attention. Yashima et al. have performed detailed investigations of Pr2(Ni0.75Cu0.25)1−xGaxO4+y (x = 0, 0.05) and Sr2Ti0.9Co0.1O4−y in order to investigate the effect of the different dopants and oxygen content on the performance.140 The Ga-doped phase had the highest interstitial oxide ion content and oxygen permeation rate, and nuclear density distribution maps showed the existence of an interstitial diffusion mechanism. Oxygen and water incorporation has been reported in Pr2−xSrxNiO4+y (x ≤ 0.5) by Grimaud et al.141 The results confirmed the incorporation of water into Pr2NiO4+y, and showed that Sr incorporation eliminates the ability to incorporate water and reduces the level of oxygen excess (y) achievable.
Chemical compatibility studies of La2NiO4+y with a Gd0.1Ce0.9O1.95 electrolyte have been examined by Sayers et al. using high resolution synchrotron X-ray diffraction studies.142 The results showed that the reactivity was dependent on the oxygen partial pressure, with studies of composite samples in a sealed capillary environment showing a reaction involving decomposition of La2NiO4+y to La2O3 and Ni, whereas no reaction was observed when the heat treatment was performed open to ambient air.
In other work on K2NiF4-type systems, computer modelling studies of oxide ion diffusion mechanisms in La2−xSrxCoO4±y have been performed.143 The work suggested that in samples with high Sr contents, oxide ion migration occurs via a vacancy mechanism within the perovskite layers, while for lower Sr contents, where oxide ion excess is present, an interstitial mechanism within the ab-plane occurs.
In terms on non-perovskite systems, improved stability of ABaCo4−xZnxO7 (1 ≤ x ≤ 2; A = In, Y) cathode materials has been reported through the introduction of both In and Y on the A site in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.144 The best performance in terms of electrochemical performance, thermal expansion match and chemical compatibility in conjunction with Gd0.2Ce0.8O1.9 electrolyte, was observed for the phase with composition Y0.5In0.5BaCo3ZnO7.
1 ratio.144 The best performance in terms of electrochemical performance, thermal expansion match and chemical compatibility in conjunction with Gd0.2Ce0.8O1.9 electrolyte, was observed for the phase with composition Y0.5In0.5BaCo3ZnO7.
Promising performance has also been observed for a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 wt% composite of Ba2Co9O14 and Sm0.2Ce0.8O1.9 on a doped LaGaO3 electrolyte.145 Good cathode performance was observed for the composite, and this system in conjunction with a Ni-Gd doped CeO2 anode gave a maximum power density of 0.45 W cm−2 at 800 °C.
50 wt% composite of Ba2Co9O14 and Sm0.2Ce0.8O1.9 on a doped LaGaO3 electrolyte.145 Good cathode performance was observed for the composite, and this system in conjunction with a Ni-Gd doped CeO2 anode gave a maximum power density of 0.45 W cm−2 at 800 °C.
2.3 Anode materials
Cermets of Ni and the electrolyte continue to be the preferred anode in SOFC systems, but there has been growing evidence to suggest that rare earth-doped CeO2 additions improve the anode performance. Chueh et al. have performed detailed studies to investigate the importance of such doped ceria additions in Ni, Pt cermet anodes.146 Through the use of well defined microstructures, the authors were able to confirm the importance of the Sm-doped CeO2 in the electrocatalysis of the hydrogen oxidation reaction, suggesting that controlling the microstructure of the CeO2 based oxide may be more important than the traditional approach of maximising the number of three phase boundaries. In terms of other fundamental studies, there is growing realisation of the importance of interfaces between electrodes and electrolytes in SOFCs. In this area, the interface between Ni and LaNbO4 proton conductors has been investigated by Kepaptsoglou et al.147 Through electron energy loss spectroscopy (EELS), the authors showed no evidence for reaction or interdiffusion layers at the interface. Ab initio calculations predicted direct bonds between the Ni and LaNbO4 phase, consistent with the EELS results, suggesting some charge transfer across the interface.Perovskite oxides have also attracted significant interest due to potential benefits through redox stability, and stability towards fuel gas impurities. Irvine and coworkers have extended prior studies on A site deficient perovskite titanate anodes, examining a Ca- and Sr-co-doped system, La0.2Sr0.25Ca0.45TiO3.148 After impregnation with both ceria and Ni, significantly improved performance was observed. Moreover on redox-cycling further improvements were observed showing an interesting strategy for improving performance.
Detailed structural studies of the perovskite phase, SrFe0.75Mo0.25O3−y, have been reported by Retuerto et al.149 Powder neutron diffraction studies indicated a tetragonal rather than the previously reported orthorhombic cell, and XAS and Mössbauer results indicated oxidation states of +3, +4 (for Fe) and +6 (for Mo). Higher Mo content phases, SrMo1−xFexO3−y (x ≤ 0.3) have been analysed by Martinez-Coronado et al.150 Neutron diffraction studies showed that all systems were cubic at room temperature, with the transition to lower symmetry tetragonal-, and then orthorhombic-cells as the temperature was reduced below room temperature. In terms of other Fe-containing systems, Haag et al. have investigated composites of LaSr2Fe2CrO9 with Gd0.1Ce0.9O1.95 in conjunction with a doped LaGaO3 based electrolyte (with protective CeO2 based layer between the electrolyte and anode).151 Good performance (>0.4 W cm−2) was obtained at 800 °C using humidified H2 as the fuel, and the anode was shown to be stable towards 22 ppm H2S impurities, albeit degrading at higher concentrations.
Doping BaIn0.3Ti0.7O2.85 electrolytes with Fe and La has been examined by Moser et al.,152 with the results showing an improvement in the total conductivity; a conductivity of 3 S cm−1 was observed for Ba0.7La0.3In0.3Ti0.1Fe0.6O3−y in air, although in reducing conditions the conductivities were 2 orders of magnitude lower. Use of this material as part of a cermet with Ni was shown to give better performance than a Ni/BaIn0.3Ti0.7O2.85 cermet.
There have been further studies of CeVO4 anode systems which convert to conductive perovskite on reduction. Partial substitution of Ce by transition metals (Ni, Co, Cu) has been analysed by Adijanto et al.153 On reduction, the dopants were shown to move out of the lattice to produce metal nanoparticles on the surface leading to good anode performance. Further improvements could be made through co-doping with Sr.
2.4 Electrolysers
In terms of electrolyser work, Gan et al. have examined the performance of a (La0.75Sr0.25)0.95Mn0.5Cr0.5O3−y–BaCe0.5Zr0.3Y0.16Zn0.04O3−y composite oxygen electrode in a solid oxide electrolyser utilising a proton conducting electrolyte.154 An electrode polarisation resistance of 0.1 Ω cm2 was obtained and stable short term steam electrolysis performance (applied voltage = 2 V, at 700 °C) was demonstrated with a current efficiency of 22%.References
- D. Capsoni, M. Bini, S. Ferrari, E. Quartarone and P. Mustarelli, J. Power Sources, 2012, 220, 253–263 CrossRef CAS.
- Q. Sun, Y. Yang and Z.-W. Fu, Electrochem. Commun., 2012, 16, 22–25 CrossRef CAS.
- Y. Kim, ACS Appl. Mater. Interfaces, 2012, 4, 2329–2333 CAS.
- Y. Deng, S. Liu and X. Liang, J. Solid State Electrochem., 2012, 17, 1067–1075 CrossRef.
- K.-C. Jiang, X.-L. Wu, Y.-X. Yin, J.-S. Lee, J. Kim and Y.-G. Guo, ACS Appl. Mater. Interfaces, 2012, 4, 4858–4863 CAS.
- C. Yu, G. Li, X. Guan, J. Zheng, L. Li and T. Chen, Electrochim. Acta, 2012, 81, 283–291 CrossRef CAS.
- H. Yu, H. Kim, Y. Wang, P. He, D. Asakura, Y. Nakamura and H. Zhou, Phys. Chem. Chem. Phys., 2012, 14, 6584 RSC.
- H. Z. Zhang, Q. Q. Qiao, G. R. Li, S. H. Ye and X. P. Gao, J. Mater. Chem., 2012, 22, 13104 RSC.
- C. R. Fell, M. Chi, Y. S. Meng and J. L. Jones, Solid State Ionics, 2012, 207, 44–49 CrossRef CAS.
- G. Singh, W. C. West, J. Soler and R. S. Katiyar, J. Power Sources, 2012, 218, 34–38 CrossRef CAS.
- Y. Dong, Z. Wang, H. Qin and X. Sui, RSC Adv., 2012, 2, 11988 RSC.
- J.-H. Cho, J.-H. Park, M.-H. Lee, H.-K. Song and S.-Y. Lee, Energy Environ. Sci., 2012, 5, 7124 CAS.
- G. Zhao, Y. Lin, T. Zhou, Y. Lin, Y. Huang and Z. Huang, J. Power Sources, 2012, 215, 63–68 CrossRef CAS.
- S. J. R. Prabakar, S. C. Han, S. P. Singh, D. K. Lee, K.-S. Sohn and M. Pyo, J. Power Sources, 2012, 209, 57–64 CrossRef CAS.
- M. Dahbi, S. Urbonaite and T. Gustafsson, J. Power Sources, 2012, 205, 456–462 CrossRef CAS.
- H. Hao, J. Wang, J. Liu, T. Huang and A. Yu, J. Power Sources, 2012, 210, 397–401 CrossRef CAS.
- B. Huang, X. Zheng and M. Lu, J. Alloys Compd., 2012, 525, 110–113 CrossRef CAS.
- X. Wu, X. Jiang, Q. Huo and Y. Zhang, Electrochim. Acta, 2012, 80, 50–55 CrossRef CAS.
- M. Zhang, Q. Chen, Z. Xi, Y. Hou and Q. Chen, J. Mater. Sci., 2012, 47, 2328–2332 CrossRef CAS.
- C. Dippel, S. Krueger, R. Kloepsch, P. Niehoff, B. Hoffmann, S. Nowak, S. Passerini, M. Winter and J. Li, Electrochim. Acta, 2012, 85, 66–71 CrossRef CAS.
- R. J. Gummow, N. Sharma, V. K. Peterson and Y. He, J. Power Sources, 2012, 197, 231–237 CrossRef CAS.
- T. Kawase and H. Yoshitake, Microporous Mesoporous Mater., 2012, 155, 99–105 CrossRef CAS.
- D. M. Kempaiah, D. Rangappa and I. Honma, Chem. Commun., 2012, 48, 2698 RSC.
- R. C. Longo, K. Xiong, W. Wang and K. Cho, Electrochim. Acta, 2012, 80, 84–89 CrossRef CAS.
- M. Reynaud, M. Ati, B. C. Melot, M. T. Sougrati, G. Rousse, J.-N. Chotard and J.-M. Tarascon, Electrochem. Commun., 2012, 21, 77–80 CrossRef CAS.
- S. C. Chung, P. Barpanda, S. Nishimura, Y. Yamada and A. Yamada, Phys. Chem. Chem. Phys., 2012, 14, 8678 RSC.
- A. V Radha, J. D. Furman, M. Ati, B. C. Melot, J. M. Tarascon and A. Navrotsky, J. Mater. Chem., 2012, 22, 24446 RSC.
- L. Damen, F. De Giorgio, S. Monaco, F. Veronesi and M. Mastragostino, J. Power Sources, 2012, 218, 250–253 CrossRef CAS.
- A. Kulka, A. Milewska, W. Zając, K. Świerczek, E. Hanc and J. Molenda, Solid State Ionics, 2012, 225, 575–579 CrossRef CAS.
- Y.-U. Park, D.-H. Seo, B. Kim, K.-P. Hong, H. Kim, S. Lee, R. A. Shakoor, K. Miyasaka, J.-M. Tarascon and K. Kang, Sci. Rep., 2012, 2, 704 Search PubMed.
- Y. Jiang, W. Xu, D. Chen, Z. Jiao, H. Zhang, Q. Ma, X. Cai, B. Zhao and Y. Chu, Electrochim. Acta, 2012, 85, 377–383 CrossRef CAS.
- A. Basa, E. Gonzalo, A. Kuhn and F. Garca-Alvarado, J. Power Sources, 2012, 207, 160–165 CrossRef CAS.
- B. Hu, X. Wang, Y. Wang, Q. Wei, Y. Song, H. Shu and X. Yang, J. Power Sources, 2012, 218, 204–211 CrossRef CAS.
- J. Kohl, D. Wiedemann, S. Nakhal, P. Bottke, N. Ferro, T. Bredow, E. Kemnitz, M. Wilkening, P. Heitjans and M. Lerch, J. Mater. Chem., 2012, 22, 15819 RSC.
- M. Nagarathinam, K. Saravanan, E. J. H. Phua, M. V Reddy, B. V. R. Chowdari and J. J. Vittal, Angew. Chem., Int. Ed., 2012, 51, 5866–5870 CrossRef CAS.
- L. Bai, J. Zhu, X. Zhang and Y. Xie, J. Mater. Chem., 2012, 22, 16957 RSC.
- A. Bhaskar, M. Deepa, T. N. Rao and U. V Varadaraju, J. Power Sources, 2012, 216, 169–178 CrossRef CAS.
- S. L. Cheekati, Z. Yao and H. Huang, J. Nanomater., 2012, 2012, 1–10 CrossRef.
- M. Du, C. Xu, J. Sun and L. Gao, Electrochim. Acta, 2012, 80, 302–307 CrossRef CAS.
- A. K. Rai, J. Gim, J. Song, V. Mathew, L. T. Anh and J. Kim, Electrochim. Acta, 2012, 75, 247–253 CrossRef CAS.
- D. Cai, P. Lian, X. Zhu, S. Liang, W. Yang and H. Wang, Electrochim. Acta, 2012, 74, 65–72 CrossRef CAS.
- J. C. Perez-Flores, F. Garcia-Alvarado, M. Hoelzel, I. Sobrados, J. Sanz and A. Kuhn, Dalton Trans., 2012, 41, 14633 RSC.
- W. Cho, J. H. Song, J.-H. Kim, G. Jeong, E. Y. Lee and Y.-J. Kim, J. Appl. Electrochem., 2012, 42, 909–915 CrossRef CAS.
- Y. Deng, Q. Zhang, Z. Shi, L. Han, F. Peng and G. Chen, Electrochim. Acta, 2012, 76, 495–503 CrossRef CAS.
- F. M. Courtel, Y. Abu-Lebdeh and I. J. Davidson, Electrochim. Acta, 2012, 71, 123–127 CrossRef CAS.
- L. Hu, P. Zhang, H. Zhong, X. Zheng, N. Yan and Q. Chen, Chem.–Eur. J., 2012, 18, 15049–15056 CrossRef CAS.
- Y. Ding, Y. Yang and H. Shao, Solid State Ionics, 2012, 217, 27–33 CrossRef CAS.
- S. Yoon, K.-N. Jung, C. S. Jin and K.-H. Shin, J. Alloys Compd., 2012, 536, 179–183 CrossRef CAS.
- W.-J. Cui, J. Yi, L. Chen, C.-X. Wang and Y.-Y. Xia, J. Power Sources, 2012, 217, 77–84 CrossRef CAS.
- H. S. Lee, J. Choi, J. Jin, J. Chun, S. M. Lee, H. J. Kim and S. U. Son, Chem. Commun., 2012, 48, 94 RSC.
- X. Wu, J. Miao, W. Han, Y.-S. Hu, D. Chen, J.-S. Lee, J. Kim and L. Chen, Electrochem. Commun., 2012, 25, 39–42 CrossRef CAS.
- Y. Zeng, W. Zhang, C. Xu, N. Xiao, Y. Huang, D. Y. W. Yu, H. H. Hng and Q. Yan, Chem.–Eur. J., 2012, 18, 4026–4030 CrossRef CAS.
- Y. Xu, Y. Li, S. Liu, H. Li and Y. Liu, J. Power Sources, 2012, 220, 103–107 CrossRef CAS.
- L. Li, F. Meng and S. Jin, Nano Lett., 2012, 12, 6030–6037 CrossRef CAS.
- L. Liu, M. Zhou, L. Yi, H. Guo, J. Tan, H. Shu, X. Yang, Z. Yang and X. Wang, J. Mater. Chem., 2012, 22, 17539 RSC.
- E. Rangasamy, J. Wolfenstine, J. Allen and J. Sakamoto, J. Power Sources, 2013, 320, 377–383 Search PubMed.
- J. L. Allen, J. Wolfenstine, E. Rangasamy and J. Sakamoto, J. Power Sources, 2012, 206, 315–319 CrossRef CAS.
- H. Buschmann, S. Berendts, B. Mogwitz and J. Janek, J. Power Sources, 2012, 206, 236–244 CrossRef CAS.
- M. Huang, A. Dumon and C.-W. Nan, Electrochem. Commun., 2012, 21, 62–64 CrossRef CAS.
- J. Wolfenstine, J. Ratchford, E. Rangasamy, J. Sakamoto and J. L. Allen, Mater. Chem. Phys., 2012, 134, 571–575 CrossRef CAS.
- I. Kokal, K. V Ramanujachary, P. H. L. Notten and H. T. Hintzen, Mater. Res. Bull., 2012, 47, 1932–1935 CrossRef CAS.
- F. Ding, W. Xu, Y. Shao, X. Chen, Z. Wang, F. Gao, X. Liu and J.-G. Zhang, J. Power Sources, 2012, 214, 292–297 CrossRef CAS.
- P. Yadav and M. C. Bhatnagar, Ceram. Int., 2012, 38, 1731–1735 CrossRef CAS.
- S. Ohta, T. Kobayashi, J. Seki and T. Asaoka, J. Power Sources, 2012, 202, 332–335 CrossRef CAS.
- B. L. Ellis and L. F. Nazar, Curr. Opin. Solid State Mater. Sci., 2012, 16, 168–177 CrossRef CAS.
- V. Palomares, P. Serras, I. Villaluenga, K. B. Hueso, J. Carretero-Gonzalez and T. Rojo, Energy Environ. Sci., 2012, 5, 5884 CAS.
- J. F. Whitacre, T. Wiley, S. Shanbhag, Y. Wenzhuo, A. Mohamed, S. E. Chun, E. Weber, D. Blackwood, E. Lynch-Bell, J. Gulakowski, C. Smith and D. Humphreys, J. Power Sources, 2012, 213, 255–264 CrossRef CAS.
- P. Barpanda, T. Ye, S. Nishimura, S.-C. Chung, Y. Yamada, M. Okubo, H. Zhou and A. Yamada, Electrochem. Commun., 2012, 24, 116–119 CrossRef CAS.
- Z. Jian, L. Zhao, H. Pan, Y.-S. Hu, H. Li, W. Chen and L. Chen, Electrochem. Commun., 2012, 14, 86–89 CrossRef CAS.
- J. Kang, S. Baek, V. Mathew, J. Gim, J. Song, H. Park, E. Chae, A. K. Rai and J. Kim, J. Mater. Chem., 2012, 22, 20857–20860 RSC.
- S.-M. Oh, S.-T. Myung, J. Hassoun, B. Scrosati and Y.-K. Sun, Electrochem. Commun., 2012, 22, 149–152 CrossRef CAS.
- A. Sun, F. R. Beck, D. Haynes, J. A. Poston Jr., S. R. Narayanan, P. N. Kumta and A. Manivannan, Mater. Sci. Eng., B, 2012, 177, 1729–1733 CrossRef CAS.
- D. Kim, E. Lee, M. Slater, W. Lu, S. Rood and C. S. Johnson, Electrochem. Commun., 2012, 18, 66–69 CrossRef CAS.
- E. Hosono, T. Saito, J. Hoshino, M. Okubo, Y. Saito, D. Nishio-Hamane, T. Kudo and H. Zhou, J. Power Sources, 2012, 217, 43–46 CrossRef CAS.
- S. Rasul, S. Suzuki, S. Yamaguchi and M. Miyayama, Electrochim. Acta, 2012, 82, 243–249 CrossRef CAS.
- A. Kitajou, H. Komatsu, K. Chihara, I. D. Gocheva, S. Okada and J. Yamaki, J. Power Sources, 2012, 198, 389–392 CrossRef CAS.
- R. A. Shakoor, S. Y. Lim, H. Kim, K.-W. Nam, J. K. Kang, K. Kang and J. W. Choi, Solid State Ionics, 2012, 218, 35–40 CrossRef CAS.
- H. Lee, Y.-I. Kim, J.-K. Park and J. W. Choi, Chem. Commun., 2012, 48, 8416–8418 RSC.
- Y. Lu, L. Wang, J. Cheng and J. B. Goodenough, Chem. Commun., 2012, 48, 6544–6546 RSC.
- S. Komaba, Y. Matsuura, T. Ishikawa, N. Yabuuchi, W. Murata and S. Kuze, Electrochem. Commun., 2012, 21, 65–68 CrossRef CAS.
- J. W. Fergus, Solid State Ionics, 2012, 227, 102–112 CrossRef CAS.
- M. Reynaud, P. Barpanda, G. Rousse, J.-N. Chotard, B. C. Melot, N. Recham and J.-M. Tarascon, Solid State Sci., 2012, 14, 15–20 CrossRef CAS.
- A. Aguadero, L. Fawcett, S. Taub, R. Woolley, K.-T. Wu, N. Xu, J. A. Kilner and S. J. Skinner, J. Mater. Sci., 2012, 47, 3925–3948 CrossRef CAS.
- M. A. Laguna-Bercero, J. Power Sources, 2012, 203, 4–16 CrossRef CAS.
- E. Fabbri, L. Bi, D. Pergolesi and E. Traversa, Adv. Mater., 2012, 24, 195–208 CrossRef CAS.
- C.-T. Chen, S. Sen and S. Kim, Chem. Mater., 2012, 24, 3604–3609 CrossRef CAS.
- M. Burbano, S. T. Norberg, S. Hull, S. G. Eriksson, D. Marrocchelli, P. A. Madden and G. W. Watson, Chem. Mater., 2012, 24, 222 CrossRef CAS.
- S. P. Miller, B. I. Dunlap and A. S. Fletcher, Solid State Ionics, 2012, 227, 66–72 CrossRef CAS.
- L. Ge, R. Li, S. He, H. Chen and L. Guo, Int. J. Hydrogen Energy, 2012, 37, 16123–16129 CrossRef CAS.
- C. Tande, D. Perez-Coll and G. C. Mather, J. Mater. Chem., 2012, 22, 11208–11213 RSC.
- J. Huang, L. Zhang, C. Wang and P. Zhang, Int. J. Hydrogen Energy, 2012, 37, 13044 CrossRef CAS.
- K. T. Lee, H. S. Yoon and E. D. Wachsman, J. Mater. Res., 2012, 27, 2063–2078 CrossRef CAS.
- K. Fukuda, T. Asaka, M. Oyabu, D. Urushihara, A. Berghout, E. Bechade, O. Masson, I. Julien and P. Thomas, Chem. Mater., 2012, 24, 4623–4631 CrossRef CAS.
- K. Fukuda, T. Asaka, N. Ishizawa, H. Mino, D. Urushihara, A. Berghout, E. Bechade, O. Masson, I. Julien and P. Thomas, Chem. Mater., 2012, 24, 2611–2618 CrossRef CAS.
- K. Matsunaga and K. Toyoura, J. Mater. Chem., 2012, 22, 7265–7273 RSC.
- H. Kiyono, Y. Matsuda, T. Shimada, M. Ando, I. Oikawa, H. Maekawa, S. Nakayama, S. Ohki, M. Tansho, T. Shimizu, P. Florian and D. Massiot, Solid State Ionics, 2012, 228, 64–69 CrossRef CAS.
- Y. Wei and M. Z. Yates, Chem. Mater., 2012, 24, 1738–1743 CrossRef.
- P. Singh and J. B. Goodenough, Energy Environ. Sci., 2012, 5, 9626–9631 CAS.
- M. T. Caldes, K. V. Kravchyk, M. Benamira, N. Besnard, V. Gunes, O. Bohnke and O. Joubert, Chem. Mater., 2012, 24, 4641–4646 CrossRef CAS.
- J. Li, H. Yoon, T.-K. Oh and E. D. Wachsman, Int. J. Hydrogen Energy, 2012, 37, 16006–16012 CrossRef CAS.
- L. Buannic, F. Blanc, D. S. Middlemiss and C. P. Grey, J. Am. Chem. Soc., 2012, 134, 14483–14498 CrossRef CAS.
- Y. Z. Wang, A. Chesnaud, E. Bevillon and G. Dezanneau, Solid State Ionics, 2012, 214, 45–55 CrossRef CAS.
- M. Shirpour, R. Merkle and J. Maier, Solid State Ionics, 2012, 216, 1–5 CrossRef CAS.
- A. Jarry, E. Quarez, K. Kravchyk and O. Joubert, Solid State Ionics, 2012, 216, 11–14 CrossRef CAS.
- J. F. Shin, K. Joubel, D. C. Apperley and P. R. Slater, Dalton Trans., 2012, 41, 261–266 RSC.
- V. Besikiotis, C. S. Knee, I. Ahmed, R. Haugsrud and T. Norby, Solid State Ionics, 2012, 228, 1–7 CrossRef CAS.
- M. Amsif, A. Magraso, D. Marrero-Lopez, J. C. Ruiz-Morales, J. Canales-Vazquez and P. Nunez, Chem. Mater., 2012, 24, 3868–3877 CrossRef CAS.
- Y. Huang, Q. Li, A. H. Jensen, M. Yin, J. O. Jensen, E. Christensen, C. Pan, N. J. Bjerrum and W. Xing, J. Mater. Chem., 2012, 22, 22452–22458 RSC.
- Y. Shen, P. Heo, C. Pak, H. Chang and T. Hibino, Electrochem. Commun., 2012, 24, 82–84 CrossRef CAS.
- Y.-M. Kim, J. He, M. D. Biegalski, H. Ambaye, V. Lauter, H. M. Christen, S. T. Pantelides, S. J. Pennycook, S. V. Kalinin and A. Y. Borisevich, Nat. Mater., 2012, 11, 888–894 CrossRef CAS.
- P. Mueller, H. Stormer, L. Dieterle, C. Niedrig, E. Ivers-Tiffee and D. Gerthsen, Solid State Ionics, 2012, 206, 57–66 CrossRef CAS.
- J. M. Porras-Vazquez and P. R. Slater, Fuel Cells, 2012, 209, 180–183 CAS.
- L. Bi, E. Fabbri and E. Traversa, Solid State Ionics, 2012, 214, 1–5 CrossRef CAS.
- H. Yung, L. Jian and S. P. Jiang, J. Electrochem. Soc., 2012, 159, F794–F798 CrossRef CAS.
- Y. Chen, D. Chen, R. Ran, H. J. Park, C. Kwak, S. J. Ahn, K. S. Moon and Z. Shao, Electrochem. Commun., 2012, 14, 36–38 CrossRef CAS.
- A. Aguadero, D. Perez-Coll, J. A. Alonso, S. J. Skinner and J. Kilner, Chem. Mater., 2012, 24, 2655–2663 CrossRef CAS.
- S. Ricote, N. Bonanos, F. Lenrick and R. Wallenberg, J. Power Sources, 2012, 218, 313–319 CrossRef CAS.
- A. J. Samson, M. Sogaard and N. Bonanos, Electrochem. Solid-State Lett., 2012, 15, B54–B56 CrossRef CAS.
- V. B. Vert, J. M. Serra, J. A. Kilner and M. Burriel, J. Power Sources, 2012, 213, 270–274 CrossRef CAS.
- T. Hong, L. Zhang, F. L. Chen and C. R. Xia, J. Power Sources, 2012, 218, 254–260 CrossRef CAS.
- Q. Zhou, W. C. J. Wei, Y. Guo and D. Jia, Electrochem. Commun., 2012, 19, 36–38 CrossRef CAS.
- Y. Li, J. Cheng, J. Song, J. A. Alonso, M. T. Fernandez-Diaz and J. B. Goodenough, Chem. Mater., 2012, 24, 4114–4122 CrossRef CAS.
- M. Burriel, J. Pena-Martinez, R. J. Chater, S. Fearn, A. V. Berenov, S. J. Skinner and J. A. Kilner, Chem. Mater., 2012, 24, 613–621 CrossRef CAS.
- Y. Hu, O. Hernandez, T. Broux, M. Bahout, J. Hermet, A. Ottochian, C. Ritter, G. Geneste and G. Dezanneau, J. Mater. Chem., 2012, 22, 18744–18747 RSC.
- R. Martinez-Coronado, A. Aguadero, D. Perez-Coll, L. Troncoso, J. A. Alonso and M. T. Fernandez-Diaz, Int. J. Hydrogen Energy, 2012, 37, 18310–18318 CrossRef CAS.
- Y. Rao, S. Zhong, F. He, Z. Wang, R. Peng and Y. Lu, Int. J. Hydrogen Energy, 2012, 37, 12522–12527 CrossRef CAS.
- A. Grimaud, F. Mauvy, J. M. Bassat, S. Fourcade, L. Rocheron, M. Marrony and J. C. Grenier, J. Electrochem. Soc., 2012, 159, B683–B694 CrossRef CAS.
- C. Solis, L. Navarrete, S. Roitsch and J. M. Serra, J. Mater. Chem., 2012, 22, 16051–16059 RSC.
- E. Quarez, K. V. Kravchyk and O. Joubert, Solid State Ionics, 2012, 216, 19–24 CrossRef CAS.
- Y. Y. Ren, R. Kungas, R. J. Gorte and C. S. Deng, Solid State Ionics, 2012, 212, 47–54 CrossRef CAS.
- Z. J. Yang, Z. L. Ding, J. Xiao, H. M. Zhang, G. I. Ma and Z. F. Zhou, J. Power Sources, 2012, 220, 15–19 CrossRef CAS.
- F. Zhang, Z. Yang, H. Wang, W. Wang and G. Ma, Fuel Cells, 2012, 12, 749–753 CrossRef CAS.
- Y. Ding, Y. Chen, L. Yonghong and B. Lin, Int. J. Hydrogen Energy, 2012, 37, 9830–9835 CrossRef CAS.
- S. Huang, G. Wang, X. Sun, C. Lei, T. Li and C. Wang, J. Alloys Compd., 2012, 543, 26–30 CrossRef CAS.
- Z. J. Yang, N. B. Wang, J. Xiao, H. M. Zhang, F. Zhang, G. L. Ma and Z. F. Zhou, J. Power Sources, 2012, 204, 89–93 CrossRef CAS.
- J. M. Porras-Vazquez, T. F. Kemp, J. V. Hanna and P. R. Slater, J. Mater. Chem., 2012, 22, 8287–8293 RSC.
- J. M. Porras-Vazquez and P. R. Slater, Fuel Cells, 2012, 12, 1056–1063 CrossRef CAS.
- Y. Chen, W. Jung, Z. Cai, J. J. Kim, H. L. Tuller and B. Yildiz, Energy Environ. Sci., 2012, 5, 7979–7988 CAS.
- Y. Ren, J. Ma, D. Ai, X. Lin and C. Deng, Electrochem. Commun., 2012, 24, 32–34 CrossRef CAS.
- M. Yashima, H. Yamada, S. Nuansaeng and T. Ishihara, Chem. Mater., 2012, 24, 4100–4113 CrossRef CAS.
- A. Grimaud, F. Mauvy, J. M. Bassat, S. Fourcade, M. Marrony and J. C. Grenier, J. Mater. Chem., 2012, 22, 16017–16025 RSC.
- R. Sayers, J. E. Parker, C. C. Tang and S. J. Skinner, J. Mater. Chem., 2012, 22, 3536–3543 RSC.
- C. Tealdi, C. Ferrara, P. Mustarelli and M. S. Islam, J. Mater. Chem., 2012, 22, 8969–8975 RSC.
- Y. N. Kim, J. H. Kim, A. Huq, M. P. Paranthaman and A. Manthiram, J. Power Sources, 2012, 214, 7–14 CrossRef CAS.
- Y. Li, M. W. Xu and J. B. Goodenough, J. Power Sources, 2012, 209, 40–43 CrossRef CAS.
- W. C. Chueh, Y. Hao, W. Jung and S. M. Haile, Nat. Mater., 2012, 11, 155–161 CrossRef CAS.
- D.-M. Kepaptsoglou, K. Hadidi, O.-M. Lovvik, A. Magraso, T. Norby, A. E. Gunnaes, A. Olsen and Q. M. Ramasse, Chem. Mater., 2012, 24, 4152–4159 CrossRef CAS.
- M. C. Verbraeken, B. Iwanschitz, A. Mai and J. T. S. Irvine, J. Electrochem. Soc., 2012, 159, F757–F762 CrossRef CAS.
- M. Retuerto, M.-R. Li, Y. B. Go, A. Ignatov, M. Croft, K. V. Ramanujachary, J. Hadermann, J. P. Hodges, R. H. Herber, I. Nowik and M. Greenblatt, Inorg. Chem., 2012, 51, 12273–12280 CrossRef CAS.
- R. Martinez-Coronado, A. Aguadero, J. A. Alonso and M. T. Fernandez-Diaz, Mater. Res. Bull., 2012, 47, 2148–2153 CrossRef CAS.
- J. M. Haag, D. M. Bierschenk, S. A. Barnett and K. R. Poeppelmeier, Solid State Ionics, 2012, 212, 1–5 CrossRef CAS.
- F. Moser, M. T. Caldes, M. Benamira, J. M. Greneche, P. Leone and O. Joubert, J. Power Sources, 2012, 201, 103–111 CrossRef CAS.
- L. Adijanto, V. B. Padmanabhan, R. Kuengas, R. J. Gorte and J. M. Vohs, J. Mater. Chem., 2012, 22, 11396–11402 RSC.
- Y. Gan, J. Zhang, Y. Li, S. Li, K. Xie and J. T. S. Irvine, J. Electrochem. Soc., 2012, 159, F763–F767 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2013 |
