Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Design and evaluation of switchable-hydrophilicity solvents†
Jesse R.
Vanderveen
,
Jeremy
Durelle
and
Philip G.
Jessop
*
Department of Chemistry, Queen's University, Kingston, Ontario, Canada K7L 3N6. E-mail: jessop@chem.queensu.ca; Fax: +1 (613) 533-6669; Tel: +1 (613) 533-3212
First published on 18th December 2013
Abstract
Switchable-hydrophilicity solvents (SHSs) are solvents that can switch reversibly between one form that is miscible with water to another that forms a biphasic mixture with water. For these SHSs, we use CO2 at 1 bar as a stimulus for triggering the transformation to the water-miscible form and removal of CO2 to achieve the reverse. We now report the identification of 13 new SHSs, including the first secondary amine SHSs, and a comparison of all known SHSs in terms of safety and environmental impacts. Amines which include another functional group, especially oxygen-containing groups, are less hazardous than alkylamines. Secondary amines can have improved switching speeds relative to tertiary amines. The variety of SHSs identified suggests that amine SHSs can be designed to have ideal properties for a given application.
Introduction
The widespread use of volatile solvents contributes to a variety of health, safety, and environmental problems such as inhalation toxicity, flammability, and smog formation. It is well known that non-volatile organic solvents avoid all of these problems, but they are rarely used in industry because they cannot be distilled. Distillation is the standard method for removing solvent from product at the end of almost any chemical process that uses solvents. Industry's dependence on distillation is responsible for the continued widespread use of volatile organic solvents despite their known hazards. The use of switchable-hydrophilicity solvents (SHSs), in combination with water, has been proposed as an alternative to distillation for solvent removal that does not require the use of volatile compounds.1–3A SHS is a solvent which is poorly miscible with water in one form but completely miscible with water in another form and which can be switched between these two forms by a simple change in the system. Amidine and tertiary amine SHSs have been identified1,2 which can be switched between the two forms by the addition or removal of CO2 from the system. The change in miscibility is due to an acid–base reaction between either hydrated CO2 or carbonic acid in the carbonated water and the SHS, resulting in the hydrophilic bicarbonate salt of the protonated SHS (eqn (1)). This behaviour has been exploited as a method for removing solvent from products such as soybean oil,1 algae oil,4,5 bitumen,6 and high density polystyrene powder.2
| NR3 + H2O + CO2 ⇌ NR3H+ + HCO3− | (1) |
The first known SHSs contained amidine functional groups, but were found to be impractical solvents because they are expensive to manufacture.1,2 Eight tertiary amine SHSs were then identified which overcame this limitation.2 However, some of these SHSs have health and safety concerns associated with them, such as toxicity, volatility, or flammability, which would make them less desirable for use in an industrial setting. In this paper, we identify 13 new secondary and tertiary amine SHSs which are commercially available or easily prepared. The amines were selected in order to overcome one or more of the issues presented by previously confirmed SHSs. We compare all of the SHSs in terms of boiling point, flash point, eutrophication potential, toxicity, and effects on skin (where information is available) to identify the safest and most environmentally benign SHSs.
Before we could search for new SHSs, we needed to identify the properties of known SHSs and how they differ from compounds that are not SHS. Amines, amidines, and guanidines that have already been tested for SHS behaviour1,2 are listed in Table 1. If an organic liquid forms one phase when mixed with water before CO2 is added, the system is considered monophasic and therefore not an SHS. If an organic liquid forms two phases when mixed with water both before and after CO2 is added, the system is considered biphasic. If the mixture of organic compound and water forms two phases before CO2 is added and forms one phase after CO2 is added, it is an SHS. Some guanidines formed biphasic mixtures with water initially and became monophasic upon exposure to CO2, but could not be reverted to biphasic mixtures, presumably because guanidines were far too basic. Compounds which displayed this behaviour are considered irreversible and were therefore rejected. The results of these tests are highly dependent on the proportions of water and organic solvent. The results shown in Table 1 were reported for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v
1 (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) mixtures of water to amine or 2
v) mixtures of water to amine or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v
1 (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) mixtures of water to amidine or guanidines.
v) mixtures of water to amidine or guanidines.
| Behaviour | Compound | Ratio of compound to water (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) v) |
Log Kow![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
pKaH |
|---|---|---|---|---|
| a Predicted using ALOGPS software version 2.1.17–19 b Estimated to have a pKaH similar to 1,8-diazabicycloundec-7-ene. c Estimated to have a pKaH similar to N,N,N′,N′-tetramethylguanidine. d Estimated to have a pKaH similar to tributylamine (pKaH 10.89).10 | ||||
| Monophasic | Triethanolamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−1.51 | 7.857 |
| Monophasic | N,N,N′,N′-Tetramethylethylenediamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.21 | 9.28 |
| Monophasic | N,N,N′,N′-Tetramethylguanidine | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.30 | 13.69 |
| Monophasic | N-Ethylmorpholine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.30 | 7.7010 |
| Monophasic | 1,8-Diazabicycloundec-7-ene | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.73 | 129 |
| Monophasic | N-Hexyl-N′,N′-dimethylacetamidine | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.94 | 12b |
| Irreversible | N′′-Hexyl-N,N,N′,N′-tetramethylguanidine | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.82 | 13.6c |
| Irreversible | N′′-Butyl-N,N,N′,N′-tetraethylguanidine | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.52 | 13.6c |
| Irreversible | N′′-Hexyl-N,N,N′,N′-tetraethylguanidine | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4.43 | 13.6c |
| Switchable | Triethylamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.47 | 10.6811 |
| Switchable | N,N-Dimethylbutylamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.60 | 10.0212 |
| Switchable | N-Ethylpiperidine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.75 | 10.457 |
| Switchable | N-Methyldipropylamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.96 | 10.413 |
| Switchable | N,N-Dimethylcyclohexylamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.04 | 10.4814 |
| Switchable | N-Butylpyrrolidine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.15 | 10.3615 |
| Switchable | N,N-Diethylbutylamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.37 | 10.51 |
| Switchable | N,N-Dimethylhexylamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.51 | 10.18 |
| Switchable | N,N,N′-Tripropylbutanamidine | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4.20 | 12b |
| Switchable | N,N,N′-Tributylpentanamidine | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5.99 | 12b |
| Biphasic | N,N-Dimethylaniline | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.11 | 5.0614 |
| Biphasic | N,N-Diisopropylethylamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.28 | 11.016 |
| Biphasic | Tripropylamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.83 | 10.7010 |
| Biphasic | N′′-Hexyl-N,N,N′,N′-tetrabutylguanidine | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.91 | 13.6c |
| Biphasic | Trioctylamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9.45 | 10.9d |
Results and discussion
Selecting amines for switchable behaviour
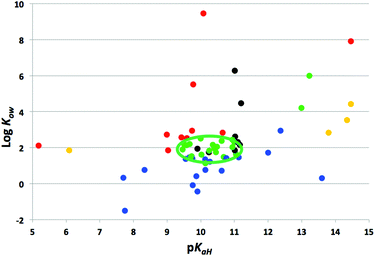
A variety of new amines were tested, but not all of them displayed SHS behaviour. Fig. 1 plots all the amines and amidines tested in this study and previous studies1,2 by the log of their octanol–water partition coefficient (log Kow) and the strength of their conjugate acids (pKaH). A trend was observed for the amines tested. First, the amine must have a log Kow between approximately 1.2 and 2.5 in order to be a SHS. Amines with lower log Kow were too hydrophilic and formed monophasic mixtures with water in their neutral form. Amines with higher log Kow were too hydrophobic and formed biphasic mixtures with water even after exposure to CO2. This trend has been observed for previously identified tertiary amine SHSs.2 Also, most amines that displayed switchable miscibility with water had pKaH above 9.5. If an amine has insufficient basicity, it will not react with carbonated water enough for a switch from a biphasic to a monophasic mixture. Although SHSs met these criteria, some amines which were not SHSs met these criteria as well, suggesting that these are necessary but not sufficient requirements for switchable behaviour. The two amidine SHSs did not fit these criteria (see the upper right portion of Fig. 1), and yet behaved as SHSs for reasons which are unclear.Amines with high boiling and flash points
Non-volatile SHSs can be designed to capitalize on the previously described advantages of SHS separations. In order to reduce volatility, SHSs with large molecular weights are preferred, but increasing the molecular weights by simply extending the alkyl chains would increase the log Kow excessively so that the bicarbonate salt of the amine would not be sufficiently soluble in carbonated water and the amine will therefore not be a SHS. By including hydrophilic functional groups in the structure of an amine while increasing the length of the alkyl chains, the solvent can be tailored to be less volatile and yet still fit within the log Kow range required for SHS behaviour.Hydrophilic functional groups also affect the basicity of the amine. The inductive effects of a functional group can decrease the pKaH of the amine, depending on the proximity of the group to the N centre. When designing a SHS with these functional groups, the exact positions of the amine and the electron withdrawing group must be considered so that the amine will be a sufficiently strong base to act as a SHS.
Of the tertiary amines tested which incorporated other functional groups, six formed monophasic mixtures with water, five formed biphasic mixtures with water, and six displayed switchable miscibility (Table 2). These six new SHSs all followed the log Kow and pKaH criteria suggested in Fig. 1 except for N,N-dimethylbenzylamine. At a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of water to amine, mixtures of water and N,N-dimethylbenzylamine remain biphasic even after prolonged bubbling of CO2 through solution. This behaviour is expected because N,N-dimethylbenzylamine (pKaH = 9.03) is a weaker base than most SHSs and will not be sufficiently protonated by carbonated water to form a monophasic mixture with water at a 1
1 volume ratio of water to amine, mixtures of water and N,N-dimethylbenzylamine remain biphasic even after prolonged bubbling of CO2 through solution. This behaviour is expected because N,N-dimethylbenzylamine (pKaH = 9.03) is a weaker base than most SHSs and will not be sufficiently protonated by carbonated water to form a monophasic mixture with water at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio. At a 5
1 volume ratio. At a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of water to amine, the amine displays switchable miscibility. Adding more water to the mixture increases the amount of amine in the aqueous phase enough to form a monophasic mixture after addition of CO2 without also resulting in a monophasic mixture when CO2 is removed. Thus a liquid can be a SHS at one volume ratio, but not a SHS at another volume ratio.
1 volume ratio of water to amine, the amine displays switchable miscibility. Adding more water to the mixture increases the amount of amine in the aqueous phase enough to form a monophasic mixture after addition of CO2 without also resulting in a monophasic mixture when CO2 is removed. Thus a liquid can be a SHS at one volume ratio, but not a SHS at another volume ratio.
| Behaviour | Compound | Log Kow![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
pKaH |
|---|---|---|---|
a Predicted using ALOGPS software version 2.1.17–19
b At a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of water to amine.
c At a 2 1 volume ratio of water to amine.
c At a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of water to amine. 1 volume ratio of water to amine.
|
|||
| Monophasic | N,N-Dimethylaminoethanol | −0.44 | 9.3112 |
| Monophasic | N,N-Dimethylaminopropanol | −0.08 | 9.7620 |
| Monophasic | N,N-Diethylaminoethanol | 0.41 | 9.8712 |
| Monophasic | N,N-Diethylglycine methyl ester | 0.76 | 7.75 |
| Monophasic | N,N-Diethylaminopropanol | 0.77 | 10.39 |
| Monophasic | 5-(Diethylamino)pentan-2-one | 1.21 | 10.121 |
| Monophasic | Ethyl 3-(diethylamino)propanoate | 1.40 | 9.35 |
| Switchable | Diisopropylaminoethanol | 1.16 | 10.1422 |
| Switchable | 4,4-Diethoxy-N,N-dimethylbutanamine | 1.48 | 9.83 |
| Switchable | Ethyl 4-(diethylamino)butanoate | 1.82 | 10.15 |
| Switchableb | N,N-Dimethylbenzylamine | 1.86 | 9.0311 |
| Switchablec | 5-(Dipropylamino)pentan-2-one | 2.15 | 10.15 |
| Switchable | N,N-Dimethylphenethylamine | 2.18 | 9.5123 |
| Switchable | Dibutylaminoethanol | 2.20 | 9.6724 |
| Biphasic | Propyl 3-(diethylamino)propanoate | 1.85 | 9.45 |
| Biphasic | N,N-Dibutylaminopropanol | 2.56 | 10.5 |
| Biphasic | Ethyl 3(dipropylamino)propanoate | 2.72 | 9.29 |
| Biphasic | N,N-Dibutylaminobutanol | 2.93 | 10.7 |
The different functional groups investigated were alcohols, esters, ketones, acetals, and aromatic rings, each of which will affect the pKaH of the amine differently. Alcohols placed two carbons away from a tertiary amine do not lower the amine's pKaH enough to prevent an amino alcohol from displaying SHS behaviour. Aromatic rings must also be 2 carbons away from a tertiary amine for SHS behaviour to be observed at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of water to amine. N,N-Dimethylaniline (pKaH 5.18) is not a strong enough base to have SHS behaviour, while N,N-dimethylbenzylamine (pKaH 9.03) displays SHS behaviour in a 5
1 volume ratio of water to amine. N,N-Dimethylaniline (pKaH 5.18) is not a strong enough base to have SHS behaviour, while N,N-dimethylbenzylamine (pKaH 9.03) displays SHS behaviour in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of water to amine. Finally, N,N-dimethylphenethylamine (pKaH 9.51) has SHS behaviour at a 1
1 volume ratio of water to amine. Finally, N,N-dimethylphenethylamine (pKaH 9.51) has SHS behaviour at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of water to amine. Ester groups must be 3 carbons away from a tertiary amine for an amino ester to display switchable miscibility, as evidenced by the glycine derivative and amino propanoates, which are not SHSs, and the amino butanoate, which is an SHS.
1 volume ratio of water to amine. Ester groups must be 3 carbons away from a tertiary amine for an amino ester to display switchable miscibility, as evidenced by the glycine derivative and amino propanoates, which are not SHSs, and the amino butanoate, which is an SHS.
The SHSs identified in Table 2 are less volatile than trialkylamine SHSs. The SHSs with additional functional groups all have boiling points above 180 °C and predicted flash points above 50 °C (Table 4, discussed later). By comparison, the least volatile trialkylamine SHS, N,N-dimethylcyclohexylamine, has a boiling point of 162 °C and a flash point of 41 °C. The differences between the boiling and flash points of trialkylamine SHSs and SHSs with additional functional groups shows that the design strategy for less-volatile SHSs is successful.
Secondary amines
Secondary amines have an alternate reactivity pathway which allows them to uptake CO2 faster than tertiary amines. Like amidines and tertiary amines, secondary amines can be converted to bicarbonate salts upon exposure to carbon dioxide and water, but they can also undergo a direct reaction with carbon dioxide to form ammonium carbamate salts (eqn (2)). This alternative reaction occurs faster than the bicarbonate salt formation, so secondary amine SHSs are likely to switch faster than tertiary amines.25 However, the energy and temperature required to remove CO2 from an aqueous ammonium carbamate solution is much larger than that required to remove CO2 from an ammonium bicarbonate solution.25 Therefore, using a secondary amine SHS can be more energy-intensive than using a tertiary amine SHS.| 2R2NH + CO2 ⇌ R2NH2+ + R2NCOO− | (2) |
While the increased rate of reaction of secondary amines is appealing, the higher energy cost of regeneration is not, so it is important to prevent significant formation of carbamate salts of an SHS. Sterically hindered amines are known to either not form carbamates or form destabilized carbamates which are rapidly hydrolyzed to bicarbonates.25 Carbamates may form as a kinetic product before being converted to bicarbonates, allowing for rapid uptake of CO2 without the large energy requirements for removing CO2.25 Therefore, a sterically hindered secondary amine SHS may switch rapidly without increased energy requirements.
Of the secondary amines tested for switchable behaviour, three formed monophasic mixtures with water, five formed biphasic mixtures with non-carbonated water but formed a precipitate upon exposure to CO2, and six displayed switchable miscibility (Table 3). X-ray crystallography of the precipitate formed from dibutylamine confirmed that it was the bicarbonate salt of the amine (Fig. 2). This result suggests that the bicarbonate salts of some secondary amines are not sufficiently soluble in water to make the amines useful as SHSs at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio.
1 volume ratio.
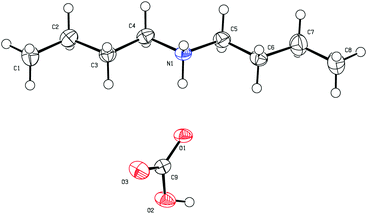 | ||
| Fig. 2 The structure of dibutylammonium bicarbonate, recrystallized from the solid formed after bubbling CO2 through a dibutylamine–water mixture. | ||
| Behaviour | Compound | Log Kow![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
pKaH |
|---|---|---|---|
a Predicted using ALOGPS software version 2.1.17–19
b Requires a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of water to amine and solution must be heated to 50 °C. 1 volume ratio of water to amine and solution must be heated to 50 °C.
|
|||
| Monophasic | Diethylamine | 0.71 | 10.927 |
| Monophasic | Ethyl 3-(tert-butylamino)propanoate | 1.38 | 10.09 |
| Monophasic | tert-Butylethylamine | 1.42 | 11.35 |
| Monophasic | Diisopropylamine | 1.46 | 11.077 |
| Switchable | Ethyl 3-(sec-butylamino)propanoate | 1.53 | 9.73 |
| Switchable | Dipropylamine | 1.64 | 11.0511 |
| Switchable | Butyl 3-(isopropylamino)propanoate | 1.90 | 9.77 |
| Switchableb | Propyl 3-(sec-butylamino)propanoate | 1.95 | 9.80 |
| Switchable | N-Propyl-sec-butylamine | 2.03 | 11.05 |
| Switchable | Di-sec-butylamine | 2.43 | 11.027 |
| Precipitates | Ethyl 3-(isobutylamino)propanoate | 1.46 | 9.45 |
| Precipitates | Ethyl 4-(tert-butylamino)butanoate | 1.75 | 10.77 |
| Precipitates | tert-Butylisopropylamine | 1.84 | 11.39 |
| Precipitates | Dibutylamine | 2.61 | 11.287 |
| Precipitates | Dihexylamine | 4.46 | 11.028 |
Increasing the temperature of the mixture or increasing the volume ratio of water to amine might result in complete dissolution of the bicarbonate salt in the water. A precipitate forms when CO2 is bubbled through a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and propyl-3-(sec-butylamino)propanoate at room temperature. If the volume ratio is adjusted to 2
1 mixture of water and propyl-3-(sec-butylamino)propanoate at room temperature. If the volume ratio is adjusted to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water to amine and the mixture is heated to 50 °C, bubbling CO2 through the mixture forms a monophasic liquid; this can be returned to a biphasic mixture if argon is bubbled through it at 65 °C. Other secondary amines which form precipitates might display SHS behaviour under different conditions. Some secondary amines, such as N-propyl-sec-butylamine, form precipitates in carbonated water at room temperature but the heat released from the exothermic reaction of the amine and carbonated water can warm the solution enough to dissolve the bicarbonate salts completely.
1 water to amine and the mixture is heated to 50 °C, bubbling CO2 through the mixture forms a monophasic liquid; this can be returned to a biphasic mixture if argon is bubbled through it at 65 °C. Other secondary amines which form precipitates might display SHS behaviour under different conditions. Some secondary amines, such as N-propyl-sec-butylamine, form precipitates in carbonated water at room temperature but the heat released from the exothermic reaction of the amine and carbonated water can warm the solution enough to dissolve the bicarbonate salts completely.
Six secondary amines were confirmed to display SHS behaviour. With the exception of dipropylamine, each of these secondary amine SHSs contained sec-butyl or isopropyl groups to destabilize carbamate salts. Converting bicarbonate salts of sterically hindered secondary amine SHSs to CO2 and neutral amine was achieved at 65 °C while passing N2 through solution. Dipropylamine–water mixtures became biphasic upon heating to 65 °C even without bubbling N2 through solution, but the solution became monophasic again when cooled to room temperature. Dipropylamine's temperature-dependent miscibility with carbonated water has been observed before,26 but is not the desired behaviour for a SHS. When the solution was heated to 90 °C for 2 h without N2 passing through it, it became biphasic and remained biphasic when cooled to room temperature. The increased temperature requirement to remove CO2 from the solution is consistent with the formation of carbamate salts, as expected for sterically unhindered secondary amines such as dipropylamine. 13C NMR analysis of carbonated water–dipropylamine solutions confirmed the presence of both bicarbonate salts and carbamate salts in solution, while no carbamates were observed for mixes of carbonated water with sterically-hindered secondary amine SHSs (see ESI†).
Every secondary amine SHS, except di-sec-butylamine, switched from a biphasic solution to a monophasic solution after less than 10 min of bubbling CO2 through the solution, while tertiary amines switch after 20 to 120 min. Di-sec-butylamine switched at a slower pace, comparable to tertiary amine SHSs. The two sec-butyl groups may be either decreasing the rate of carbamate formation substantially or preventing carbamate formation completely. Evidently, one branching group near the amine is enough to lower the energy requirements for removing CO2 while still allowing for a rapid switch from biphasic to monophasic solutions.
While most of these amines were only tested for one full switching cycle, we tested CyNMe2 and butyl 3-(isopropylamino)propanoate for their ability to handle multiple cycles. The former was used for many cycles without difficulty but the latter can only be used for one cycle, because of significant hydrolysis of the ester during the removal of the CO2. This problem may exist for other amino-esters as well.
Risk evaluation of SHSs
In order for SHSs to be considered for use industrially, it is important to consider their effects on health and the environment, preferably in comparison to the solvents that they would replace. In order to identify the safety and environmental effects of SHSs, the LD50 (oral, rat), boiling point, flash point, eutrophication potential (EP), and skin effects of all SHSs identified in this study and previous studies1,2 are compared in Table 4. The reported safety and environmental data reveal trends in the safety risks and environmental impacts of SHSs. We used hexane and toluene as representative conventional (non-switchable) solvents for comparison.| Substance | LD50 (oral, rat) (mg kg−1) | Boiling point (°C) | Flash point (°C) | Eutrophication potentiala | Skin effects | Log Kow![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
|---|---|---|---|---|---|---|
| Estimated values are shown in italics.a Calculated as mass equivalents of phosphate.b Predicted using ALOGPS software version 2.1.17–19c Predicted using TEST software version 4.1.29d Oral LD50 value for mice.e Boiling point at atmospheric pressure extrapolated from boiling point at reduced-pressure.f Several LD50 values have been reported for toluene.33,68–70 The reported LD50 value of 636 mg kg−1 may be the result of a miscalculation.69,70 | ||||||
| N,N,N′-Tributylpentanamidine | 4000 | 367 | 176 | 0.17 | n/a | 5.92 |
| N,N,N′-Tripropylbutanamidine | 700 | 303 | 137 | 0.18 | n/a | 4.20 |
| N,N-Dimethylcyclohexylamine | 34833 | 15934 | 4335 | 0.17 | Corrosive (1B)36 | 2.04 |
| N-Ethylpiperidine | 28037 | 12838 | 1737 | 0.17 | Corrosive (1B)37 | 1.74 |
| N-Butylpyrrolidine | 51,39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
15639 | 3539 | 0.17 | Irritant40 | 2.15 |
| N,N-Dimethylhexylamine | 500 | 14841 | 3442 | 0.17 | Corrosive (1B)42 | 2.51 |
| N,N-Dimethylbutylamine | 18843 | 9544 | −543 | 0.18 | Corrosive (1A)43 | 1.60 |
| N,N-Diethylbutylamine | 300 | 13645 | 2446 | 0.17 | Corrosive (1B)46 | 2.37 |
| N-Methyldipropylamine | 267 | 11747 | −348 | 0.18 | Corrosive (1B)48 | 1.96 |
| Triethylamine | 46033 | 8934 | −949 | 0.18 | Corrosive (1A)50 | 1.47 |
| N,N-Diisopropylaminoethanol | 94051 | 190,52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
6453 | 0.15 | Corrosive (1B)53 | 1.16 |
| N,N-Dibutylaminoethanol | 107033 | 230,54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
9555 | 0.15 | Corrosive (1B)55 | 2.15 |
| 4,4-Diethoxy-N,N-dimethylbutanamine | 2000 | 270,56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
7057 | 0.13 | n/a | 1.48 |
| Ethyl 4-(diethylamino)butanoate | 7000 | 220e | 77 | 0.13 | n/a | 1.82 |
| N,N-Dimethylphenethylamine | 300 | 21058 | 7159 | 0.16 | Irritant59 | 2.18 |
| Dipropylamine | 46033 | 10860 | 1749 | 0.18 | Corrosive (1A)61 | 1.64 |
| Di-sec-butylamine | 300 | 13562 | 2163 | 0.17 | Corrosive (1A)63 | 2.46 |
| 5-Dipropylaminopentanone | 3000 | 280e | 72 | 0.15 | n/a | 2.14 |
| Ethyl 3-(sec-butylamino)propanoate | 5000 | 210e | 93 | 0.13 | n/a | 1.53 |
| Propyl 3-(sec-butylamino)propanoate | 3000 | 220e | 89 | 0.13 | n/a | 1.94 |
| Butyl 3-(isopropylamino)propanoate | 3000 | 230e | 105 | 0.13 | n/a | 1.90 |
| N-Propyl-sec-butylamine | 300 | 12464 | 15 | 0.18 | Corrosive (1B)65 | 2.03 |
| N,N-Dimethylbenzylamine | 26533 | 18366 | 5367 | 0.16 | Corrosive (1B)67 | 1.86 |
| Toluene | 636–6400,33,68–70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f f |
11071 | 449 | 0.13 | Irritant72 | 2.49 |
| Hexane | 2871033 | 6973 | −2249 | 0.15 | Irritant74 | 3.55 |
The toxicities of SHSs were compared using oral LD50 data (rat). Many SHSs do not have reported LD50 values. In these cases, the U.S. Environmental Protection Agency's Toxicity Estimation Software Tool (TEST) was used to predict oral LD50 values.29 We find that the predicted toxicities of amines are within a factor of 3 of reported LD50 values 95% of the time (see ESI†). Despite the inherent inaccuracy of toxicity predictions, we expect that SHSs with predicted LD50 values above 2000 mg kg−1 are less toxic than SHSs with LD50 values of around 500 mg kg−1 or lower. Oxygen-containing SHSs have consistently higher LD50 values than dialkyl- and trialkylamine SHSs. Multiple different oral LD50 values have been reported for toluene. While toluene is less toxic than dialkyl- and trialkylamine SHSs, the varying reports of its LD50 and the uncertainty of the predicted LD50 values for other SHSs prevent us from drawing further conclusions. According to the LD50 data, hexane is much safer than every SHS. However, LD50 is a measure of acute toxicity, so solvents with chronic toxicity may appear safe even though they are not. For example, hexane is a known chronic neurotoxin, a serious problem that is not made evident by LD50 data.30–32
The more volatile SHSs are not advantageous over toluene in terms of inhalation toxicity but the less volatile SHSs are probably much safer than toluene. There is little data regarding this form of toxicity for the SHSs. The inhalation LC50 values (rat, 4 h) for triethylamine and dipropylamine are 4.1 g m−3 and 4.4 g m−3, respectively,33 while the corresponding values for toluene and hexane are 30.1 g m−3 and 169 g m−3, respectively.75,76 Dimethylcyclohexane has a reported LC50 (rat, 2 h) of 1.9 g m−3.33 The less-volatile SHSs may not pose an inhalation toxicity risk because their vapour pressures are much lower. For example the vapour pressure of propyl-3-(sec-butylamino)propanoate at 25 °C was estimated to be 13 Pa using a nomograph. By comparison, the vapour pressures of triethylamine, toluene, and hexane at 25 °C are 9670 Pa,77 3804 Pa,78 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 Pa,79 respectively. Propyl-3-(sec-butylamino)propanoate and other low-volatility amines are less likely to be inhaled because of their low vapour pressure, making them less of an inhalation toxicity risk than volatile solvents.
240 Pa,79 respectively. Propyl-3-(sec-butylamino)propanoate and other low-volatility amines are less likely to be inhaled because of their low vapour pressure, making them less of an inhalation toxicity risk than volatile solvents.
The smell of amines depends greatly on the volatility and structure. Butyl-3-(iso-propylamino)propanoate, the least volatile SHS, has no detectable smell. Ethyl 4-(diethylamino)butanoate has a weak but pleasant smell. The volatile amines have the usual unpleasant smell one expects of amines.
The boiling and flash points of SHSs give an indication of the volatility of the solvents. Flash points also show how flammable solvents are. If the boiling point at atmospheric pressure is not known, a value was estimated by extrapolating from a reduced-pressure boiling point. The TEST program can be used to predict flash points if the flash point of an SHS is not known. These predictions are accurate to within 8 °C, 81% of the time if the experimental flashpoint is above 20 °C (see ESI†). Almost every SHS is safer than toluene and hexane in terms of volatility and flammability. SHS containing another functional group in addition to an amine were designed to be less volatile and less flammable, and the data confirms that they are safer than other SHSs by these metrics. Under the UN globally-harmonized system of classification, these heavier SHSs would be classified as combustible rather than flammable liquids (flash points >60 °C).80
The eutrophication potential of the SHSs was calculated using a modified version of the equation described by Heijungs et al.81 replacing chemical oxygen demand with theoretical oxygen demand, which was calculated using an equation described by Baker, Milke and Mihelcic.82 The implications of these equations are that lower nitrogen content and higher oxygen content in a compound lowers its eutrophication potential. As a result, oxygen containing SHS have less eutrophication potential than other SHSs, with amino esters having the lowest potential. Indeed, amino ester SHSs have eutrophication potentials similar to toluene and lower than hexane, despite their nitrogen content.
Skin effects are another concern for SHSs; 13 of the SHSs are corrosive. In order to differentiate between different levels of corrosion, different classes of corrosion as defined by the Globally Harmonized System were used where information was available.80 A class 1A corrosive substance shows effects after 3 minutes of exposure and less than 1 hour of observation. A class 1B corrosive substance shows effects after 1 hour of exposure and less than 14 days of observation. Class 1A corrosive SHSs should be avoided. Fortunately, two of the SHSs and many conventional solvents like toluene and hexane are irritants, rather than corrosive liquids. For many of the SHSs, skin effect data is unavailable.
Bioaccumulation is not a concern for SHSs. Compounds with log Kow values below 3.5 are considered to have low bioaccumulation potential.83 All known amine SHSs have log Kow between approximately 1.2 and 2.5. The amidine SHSs would not bioaccumulate, despite their high log Kow values, because they are hydrolytically unstable and therefore would not likely persist in the environment long enough to pose a bioaccumulation risk. Some conventional solvents (e.g. hexane) have moderate bioaccumulation potential, while others have low potential (e.g. toluene).
The use of volatile solvents results in volatile organic compound emissions and contributes to smog formation.84 With regards to this environmental concern, solvents are generally expected to be more benign if they are less volatile. SHSs with additional functional groups are much less volatile than conventional solvents like hexane and toluene. While volatile SHSs like triethylamine will have no advantages over conventional solvents, the low-volatility SHSs likely have less potential to contribute to smog formation than conventional solvents.
The persistence of a solvent when it is released into the environment is another concern. Compounds can degrade by a number of different pathways and it can be difficult to predict their persistence. However, some degradation trends relating to chemical structures have been observed.85 Quaternary carbon centres, extensive branching, heterocycles, and tertiary amines tend to decrease degradability. Features that increase degradability are oxygen atoms (particularly esters), unsubstituted alkyl chains of 4 or more, and unsubstituted phenyl rings. Secondary amine SHSs are also expected to be more biodegradable than tertiary amine SHSs. Howard et al. found that tertiary amines are poorly biodegradable.86 Eide-Haugmo et al. suggest that secondary amines are more degradable than tertiary amines.87 Although amine biodegradation data is sparse and many exceptions are apparent, the literature data does support the notion that secondary amines will biodegrade more readily than tertiary amines.86–91 For example, dipropylamine is biodegradable while triethylamine is not.91,92 Not all tertiary amines will persist however. The biodegradation of N,N-dimethylcyclohexylamine is not rapid, but it is considered to be biodegradable in an aqueous environment (Zahn–Wellens test).93 The available data also indicates that compounds with quaternary carbons are more resistant to biodegradation than straight chain compounds. A common opinion is that any branching will decrease biodegradability. However, Boethling et al. report that this is an oversimplification and only extensive branching and quaternary carbons show a trend of decreasing degradability.85 Many secondary amine SHS contain a branching group to destabilize carbamate formation. An ideal secondary amine SHS would include one branching group to destabilize the carbamate product without significantly decreasing its biodegradability.
With regard to this information, secondary amino ester SHSs are expected to be the least persistent, particularly butyl 3-(isopropylamino)propanoate because it contains an n-butyl group. Tertiary amine SHSs containing other functional groups and dialkylamine SHSs are second choices, while trialkylamine SHSs will likely persist longer than the other SHSs. Toluene and hexane are both biodegradable.94,95 Trialkylamine SHSs should be more persistent than conventional solvents, but we expect secondary amine SHSs and tertiary amine SHS with a second functional group to have biodegradability comparable to or better than conventional solvents.
There are other considerations which can also be used to differentiate between SHSs. Some SHSs, such as the amino acetal, the aminoesters, and the amidines are prone to hydrolysis and are likely to degrade over time. Dipropylamine forms a stable carbamate salt and more energy must be put into the system to convert the salt back into CO2 and neutral amine. Some SHSs also require different amounts of water to display switchability. Most SHSs work at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio, but some require a 2
1 volume ratio, but some require a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or even 5
1 or even 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water–amine volume ratio. The amount of energy required to heat the water when removing CO2 increases as the amount of water increases. Some SHSs switch faster than others as well. In particular, secondary amines switch from biphasic to monophasic mixtures faster than tertiary amines. None of these factors are apparent from the information given in Table 4, but they can affect the overall viability of an SHS.
1 water–amine volume ratio. The amount of energy required to heat the water when removing CO2 increases as the amount of water increases. Some SHSs switch faster than others as well. In particular, secondary amines switch from biphasic to monophasic mixtures faster than tertiary amines. None of these factors are apparent from the information given in Table 4, but they can affect the overall viability of an SHS.
While no SHS can be identified as being the most benign, the structural features that generate the most benign SHSs can be determined from the above trends. The data available for oxygen-containing SHSs suggest that they are less toxic and less volatile than di- and tri-alkyl amine SHSs. They are also no more corrosive and have lower eutrophication potentials, making them the most benign SHSs identified according to the metrics listed in this study. However, the risk of hydrolysis, which limit the reusability of aminoesters, suggests that they may not be ideal. The amidine SHSs also have favourable safety and environmental properties apart from a larger eutrophication potential and likely corrosivity, but they are unlikely ever to be used because of their high cost of synthesis. No differences between secondary and tertiary amine SHSs are apparent from the data in Table 4, but the secondary amines are likely to be more biodegradable. Not every risk is identified in Table 4. There is insufficient data to comment on chronic toxicity or carcinogenicity. Because of these uncertainties, we do not recommend a single SHS as the most benign but rather we recommend consideration of all of their properties before one is used and more research including the identification of even better SHS.
Conclusions
Several new SHSs have been identified, including secondary amines and amines incorporating an additional functional group. Amines which display SHS behaviour typically have log Kow between 1.2 and 2.5 and pKaH above 9.5. Dimethylbenzylamine is an exception (pKaH 9.03), but is only switchable if the volume of water is much larger than the volume of amine. Secondary amines can also display switchable behaviour but can form carbamate salts and precipitate as bicarbonate salts. Secondary amine SHSs can be designed to avoid significant carbamate formation by making them sterically hindered. Amines incorporating other functional groups are more benign than other SHSs, commonly having lower toxicity, volatility, flammability, and eutrophication potential. Compared to toluene, the secondary amine ester SHSs are predicted to be safer for health and the environment in terms of flammability, smog formation, inhalation toxicity, and bioaccumulation (lower Kow). They are comparable to toluene in terms of eutrophication and possibly biodegradation. The variety of compounds identified and their different properties show that SHSs can be designed to meet the requirements of an application.Experimental
Chemicals were used as received. Amines were commercially available (Sigma-Aldrich, TCI, Fisher) except for amino propanoate/butanoate esters and amino ketones, which were synthesized and characterized as described below. Argon (99.998%) and CO2 (chromatographic grade) were purchased from Praxair.Testing for switchable behaviour
To confirm the switchable miscibility, amines were mixed with water in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio. If two phases were observed, CO2 was bubbled into the solution through a gas dispersion tube (Ace Glass, 25–50 micron porosity) for 2 h. If the mixture became monophasic, N2 was bubbled into the solution through a gas dispersion tube for 2 h while the solution was heated to 65 °C. If the mixture became biphasic again, the amine was classified as a SHS. Other volume ratios were attempted for some amines.
1 volume ratio. If two phases were observed, CO2 was bubbled into the solution through a gas dispersion tube (Ace Glass, 25–50 micron porosity) for 2 h. If the mixture became monophasic, N2 was bubbled into the solution through a gas dispersion tube for 2 h while the solution was heated to 65 °C. If the mixture became biphasic again, the amine was classified as a SHS. Other volume ratios were attempted for some amines.
Evaluation
Log Kow values were predicted using ALOGPS 2.1.17–19 pKaH values were found from literature or determined titrimetrically. Flash points, skin effects, and LD50 values were found from literature or MSDS. If flash point or LD50 values were unavailable, they were calculated using the TEST program.29 Eutrophication potentials (EP) were calculated using a variation of the method proposed by Heijungs et al., which calculates the eutrophication potential of a compound based on its molecular weight (MW), the number of phosphorus and nitrogen atoms it contains (νP and νN), and its theoretical oxygen demand (νThOD) (eqn (3) and (4)).81 Theoretical oxygen demands were calculated using the method described by Baker, Milke, and Mihelcic (eqn (5) and (6)),82 which assumes that nitrogen atoms are converted to NH3 and that all carbon atoms are completely oxidized. The reference compound for eutrophication potential is PO43−.| νtot,i = (νP,i + νN,i/16 + νThOD,i/138) | (3) |
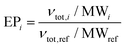 | (4) |
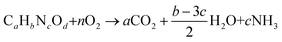 | (5) |
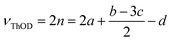 | (6) |
Dibutylammonium bicarbonate crystal formation
Dibutylamine (5 mL) and water (5 mL) were combined in a vial. CO2 was bubbled through the mixture until a large quantity of precipitate formed. The mixture was heated to 40 °C, resulting in a biphasic mixture with no solids. Upon cooling to room temperature, needle-like crystals formed at the interface between the liquid phases.Observation of secondary amine speciation in carbonated water
Amine (dipropylamine, di-sec-butylamine, or sec-butylisopropylamine, 1 mL) was mixed with 1 mL H2O in a vial and CO2 was bubbled through the solution until it became monophasic. CH3CN (0.2 mL) was added to solution as a reference compound and the solution was characterized by 13C {1H} NMR spectroscopy.Measuring the pKaH of amines
For most amines, a ∼20 mL solution containing ∼0.02 g amine in distilled water was titrated with ∼0.1 M HCl. The pH of the solution was recorded after each addition of titrant (Orion 4 Star pH meter, Thermo Scientific). The equivalence point was determined using a derivative plot and the pH at the half equivalence point was taken as the pKaH of the amine. Titrations were performed at least twice.Dibutylaminobutanol and dibutylaminopropanol were not sufficiently soluble in water to measure their aqueous pKaH directly. The pKaH of these compounds were measured in ethanol–water solutions and extrapolated to a completely aqueous solution using the method described by Gowland and Schmid.96
Synthesis
Amino esters and ketones were synthesized using procedures adapted from literature for similar compounds.97,98 NMR spectra were collected on a Bruker Avance-500 or a Bruker Avance-300 NMR spectrometer. IR spectra were collected with a Varian 640 FT-IR spectrometer. Mass Spectra were collected with a Perkin Elmer Clarus 600 T mass spectrometer connected to a Perkin Elmer Clarus 680 gas chromatograph.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1448, 1371, 1298, 1251, 1198, 1118, 1094, 1048, 984, 856, 787; m/z (EI) 173 (6), 158 (15), 144 (3), 130 (3), 116 (1), 99 (1), 86 (100), 73 (6), 72 (6), 58 (15), 56 (10), 55 (15); Anal. Calcd for C9H19NO2: C 62.39, H 11.05, N 8.09; found C 62.15, H 11.05, N 8.07. The 1H NMR data match literature values.97
O), 1448, 1371, 1298, 1251, 1198, 1118, 1094, 1048, 984, 856, 787; m/z (EI) 173 (6), 158 (15), 144 (3), 130 (3), 116 (1), 99 (1), 86 (100), 73 (6), 72 (6), 58 (15), 56 (10), 55 (15); Anal. Calcd for C9H19NO2: C 62.39, H 11.05, N 8.09; found C 62.15, H 11.05, N 8.07. The 1H NMR data match literature values.97
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1463, 1371, 1341, 1301, 1250, 1192, 1078, 1053, 855, 791; m/z (EI) 201 (9), 172 (100), 156 (2), 144 (12), 130 (4), 114 (59), 101 (6), 86 (13), 84 (27), 72 (26), 70 (12), 56 (10), 55 (26); Anal. Calcd for C11H23NO2: C 65.62, H 11.52, N 6.96; found C 65.63, H 11.60, N 6.97. The 1H NMR data matches literature values.97
O), 1463, 1371, 1341, 1301, 1250, 1192, 1078, 1053, 855, 791; m/z (EI) 201 (9), 172 (100), 156 (2), 144 (12), 130 (4), 114 (59), 101 (6), 86 (13), 84 (27), 72 (26), 70 (12), 56 (10), 55 (26); Anal. Calcd for C11H23NO2: C 65.62, H 11.52, N 6.96; found C 65.63, H 11.60, N 6.97. The 1H NMR data matches literature values.97
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1184; m/z (EI) 187 (6), 172 (3), 158 (1), 142 (12), 115 (5), 114 (6), 98 (2), 86 (100), 58 (9), 56 (5); Anal. Calcd for C10H21NO2: C 64.12, H 11.31, N 7.48; found C 64.12, H 11.44, N 7.48.
O), 1184; m/z (EI) 187 (6), 172 (3), 158 (1), 142 (12), 115 (5), 114 (6), 98 (2), 86 (100), 58 (9), 56 (5); Anal. Calcd for C10H21NO2: C 64.12, H 11.31, N 7.48; found C 64.12, H 11.44, N 7.48.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1463, 1372, 1255, 1186, 1096, 1056, 1028, 788, 734; m/z (EI) 172, 158 (7), 144 (94), 130, (5), 112 (7), 98 (65), 86 (56), 84 (7), 70 (37), 56 (100); Anal. Calcd for C9H19NO2: C 62.39, H 11.05, N 8.09; found C 62.29, H 11.29, N 8.04.
O), 1463, 1372, 1255, 1186, 1096, 1056, 1028, 788, 734; m/z (EI) 172, 158 (7), 144 (94), 130, (5), 112 (7), 98 (65), 86 (56), 84 (7), 70 (37), 56 (100); Anal. Calcd for C9H19NO2: C 62.39, H 11.05, N 8.09; found C 62.29, H 11.29, N 8.04.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1362, 1230, 1174, 1099, 102; m/z (EI), 173 (1), 158 (94), 144 (3), 130 (4), 116 (5), 112 (31), 86 (50), 70 (100), 58 (20), 57 (21), 56 (11), 55 (19); Anal. Calcd for C9H19NO2: C 62.39, H 11.05, N 8.09; found C 62.28, H 11.13, N 8.07.
O), 1362, 1230, 1174, 1099, 102; m/z (EI), 173 (1), 158 (94), 144 (3), 130 (4), 116 (5), 112 (31), 86 (50), 70 (100), 58 (20), 57 (21), 56 (11), 55 (19); Anal. Calcd for C9H19NO2: C 62.39, H 11.05, N 8.09; found C 62.28, H 11.13, N 8.07.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1467, 1372, 1254, 1186, 1121, 1061, 1029, 750; m/z (EI) 173 (3), 130 (70), 116 (7), 86 (38), 84 (100), 70 (7), 57 (13), 56 (9), 55 (9); Anal. Calcd for C9H19NO2: C 62.39, H 11.05, N 8.09; found C 62.26, H 11.23, N 8.04.
O), 1467, 1372, 1254, 1186, 1121, 1061, 1029, 750; m/z (EI) 173 (3), 130 (70), 116 (7), 86 (38), 84 (100), 70 (7), 57 (13), 56 (9), 55 (9); Anal. Calcd for C9H19NO2: C 62.39, H 11.05, N 8.09; found C 62.26, H 11.23, N 8.04.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1410, 1361, 1294, 1202, 1175, 1121, 1069, 961, 764, 714; m/z (EI) 157 (4), 142 (1), 99 (4), 86 (100), 72 (2), 71 (2), 70 (2), 58 (12), 56 (5); HRMS (EI): C9H19NO for M+ calculated 157.1467, found 157.1462.
O), 1410, 1361, 1294, 1202, 1175, 1121, 1069, 961, 764, 714; m/z (EI) 157 (4), 142 (1), 99 (4), 86 (100), 72 (2), 71 (2), 70 (2), 58 (12), 56 (5); HRMS (EI): C9H19NO for M+ calculated 157.1467, found 157.1462.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1462, 1362, 1174, 1078, 1020, 960, 746; m/z (EI) 185 (8), 156 (37), 154 (1), 140 (1), 127 (2), 114 (64), 98 (3), 85 (100), 72 (22), 70 (6), 56 (5); HRMS (EI): C11H23NO for M+ calculated 185.1780, found 185.1775.
O), 1462, 1362, 1174, 1078, 1020, 960, 746; m/z (EI) 185 (8), 156 (37), 154 (1), 140 (1), 127 (2), 114 (64), 98 (3), 85 (100), 72 (22), 70 (6), 56 (5); HRMS (EI): C11H23NO for M+ calculated 185.1780, found 185.1775.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1447, 1366, 1229, 1159, 1102, 1030, 768, 708; m/z (EI) 187 (1), 172 (100), 158 (1), 142 (3), 126 (31), 115 (25), 98 (5), 86 (82), 84 (17), 69 (17), 57 (33); Anal. Calcd for C10H21NO2: C 64.12, H 11.31, N 7.48; found C 63.94, H 11.54, N 7.47.
O), 1447, 1366, 1229, 1159, 1102, 1030, 768, 708; m/z (EI) 187 (1), 172 (100), 158 (1), 142 (3), 126 (31), 115 (25), 98 (5), 86 (82), 84 (17), 69 (17), 57 (33); Anal. Calcd for C10H21NO2: C 64.12, H 11.31, N 7.48; found C 63.94, H 11.54, N 7.47.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1463, 1377, 1356, 1259, 1186, 1099, 1063, 1002, 736; m/z (EI) 187 (1), 186 (1), 172 (8), 158 (100), 144 (1), 130 (8), 128 (6), 116 (9), 112 (8), 98 (65), 86 (60), 84 (8), 72 (15), 70 (36), 56 (98); Anal. Calcd for C10H21NO2: C 64.12, H 11.31, N 7.48; found C 64.01, H 11.54, N 7.46.
O), 1463, 1377, 1356, 1259, 1186, 1099, 1063, 1002, 736; m/z (EI) 187 (1), 186 (1), 172 (8), 158 (100), 144 (1), 130 (8), 128 (6), 116 (9), 112 (8), 98 (65), 86 (60), 84 (8), 72 (15), 70 (36), 56 (98); Anal. Calcd for C10H21NO2: C 64.12, H 11.31, N 7.48; found C 64.01, H 11.54, N 7.46.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1467, 1379, 1337, 1262, 1173, 1148, 1064, 1021, 840, 739; m/z (EI) 187 (2), 186 (2), 172 (70), 144 (14), 130 (4), 116 (13), 114 (17), 98 (64), 88 (10), 72 (100), 70 (22), 56 (89); Anal. Calcd for C10H21NO2: C 64.12, H 11.31, N 7.48; found C 64.08, H 11.45, N 7.49.
O), 1467, 1379, 1337, 1262, 1173, 1148, 1064, 1021, 840, 739; m/z (EI) 187 (2), 186 (2), 172 (70), 144 (14), 130 (4), 116 (13), 114 (17), 98 (64), 88 (10), 72 (100), 70 (22), 56 (89); Anal. Calcd for C10H21NO2: C 64.12, H 11.31, N 7.48; found C 64.08, H 11.45, N 7.49.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1465, 1382, 1266, 1198, 1178, 1062, 996, 909; m/z (EI) 187 (6), 172 (17), 158 (2), 144 (1), 130 (6), 128 (3), 100 (1), 99 (1), 98 (1), 86 (100), 73 (17), 70 (2), 58 (17), 55 (2); Anal. Calcd for C10H21NO2: C 64.12, H 11.31, N 7.48; found C 63.94, H 11.46, N 7.46.
O), 1465, 1382, 1266, 1198, 1178, 1062, 996, 909; m/z (EI) 187 (6), 172 (17), 158 (2), 144 (1), 130 (6), 128 (3), 100 (1), 99 (1), 98 (1), 86 (100), 73 (17), 70 (2), 58 (17), 55 (2); Anal. Calcd for C10H21NO2: C 64.12, H 11.31, N 7.48; found C 63.94, H 11.46, N 7.46.
Acknowledgements
We thank Switchable Solutions Inc. and the National Science and Engineering Research Council for financial support, Dr Preston Chase and Dr Lowy Gunnewiek for useful discussions, and the Canada Research Chairs program.Notes and references
- P. G. Jessop, L. Phan, A. Carrier, S. Robinson, C. J. Dürr and J. R. Harjani, Green Chem., 2010, 12, 809–814 RSC.
- P. G. Jessop, L. Kozycz, Z. G. Rahami, D. Scheonmakers, A. R. Boyd, D. Wechsler and A. M. Holland, Green Chem., 2011, 13, 619–623 RSC.
- P. G. Jessop, L. N. Phan, A. J. Carrier, R. Resendes and D. Wechsler, PCT IntlWO2011050469, 2011 Search PubMed.
- A. R. Boyd, P. Champagne, P. J. McGinn, K. M. MacDougall, J. E. Melanson and P. G. Jessop, Bioresour. Technol., 2012, 118, 628–632 CrossRef CAS PubMed.
- C. Samori, D. L. Barreiro, R. Vet, L. Pezzolesi, D. W. F. Brilman, P. Galletti and E. Tagliavini, Green Chem., 2013, 15, 353–356 RSC.
- A. Holland, D. Wechsler, A. Patel, B. M. Molloy, A. R. Boyd and P. G. Jessop, Can. J. Chem., 2012, 90, 805–810 CrossRef CAS PubMed.
- N. F. Hall and M. R. Sprinkle, J. Am. Chem. Soc., 1932, 54, 3469–3485 CrossRef CAS.
- U. Oesch, Z. Brzozka, A. Xu, B. Rusterholz, G. Suter, H. V. Pham, D. H. Welti, D. Ammann, E. Pretsch and W. Simon, Anal. Chem., 1986, 58, 2285–2289 CrossRef CAS.
- C. Wiles and P. Watts, Beilstein J. Org. Chem., 2011, 7, 1360–1371 CrossRef CAS PubMed.
- H. K. Hall, J. Phys. Chem., 1956, 60, 63–70 CrossRef CAS.
- V. Frenna, N. Vivona, G. Consiglio and D. Spinelli, J. Chem. Soc., Perkin Trans. 2, 1985, 1865–1868 RSC.
- M. M. Kreevoy and S.-W. Oh, J. Am. Chem. Soc., 1973, 95, 4805–4810 CrossRef CAS.
- R. E. McMahon and N. R. Easton, J. Med. Pharm. Chem., 1961, 4, 423–586 CrossRef.
- J. M. Devereux, K. R. Payne and E. R. A. Peeling, J. Chem. Soc., 1957, 2845–2851 RSC.
- L. C. Craig and R. M. Hixon, J. Am. Chem. Soc., 1931, 53, 4367–4372 CrossRef CAS.
- S. Jarmelo, N. Maiti, V. Anderson, P. R. Carey and R. Fausto, J. Phys. Chem. A, 2005, 109, 2069–2077 CrossRef CAS PubMed.
- I. V. Tetko and V. Y. Tanchuk, J. Chem. Inf. Comput. Sci., 2002, 42, 1136–1145 CrossRef CAS PubMed.
- I. V. Tetko, J. Gasteiger, R. Todeschini, A. Mauri, D. Livingstone, P. Ertl, V. A. Palyulin, E. V. Radchenkco, N. S. Zefirov, A. S. Makarenko, V. Y. Tanchuk and V. V. Prokopenko, J. Comput.-Aided Mol. Des., 2005, 19, 453–463 CrossRef CAS PubMed.
- VCCLAB, Virtual Computational Chemistry Laboratory, http://www.vcclab.org (accessed 22 May 2013).
- J. Hine and M. N. Khan, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1992, 31, 427–435 Search PubMed.
- G. M. Steinberg and J. Bolger, J. Am. Pharm. Assoc., 1957, 46, 188–191 CrossRef CAS.
- R. J. Littel, M. Bos and G. J. Knoop, J. Chem. Eng. Data, 1990, 35, 276–277 CrossRef CAS.
- F. Wang and L. M. Sayre, J. Am. Chem. Soc., 1992, 114, 248–255 CrossRef CAS.
- A. Gero, J. Med. Chem., 1963, 6, 457–458 CrossRef.
- F. Bougie and M. C. Iliuta, J. Chem. Eng. Data, 2012, 57, 635–669 CrossRef CAS.
- J. Zhang, R. Misch, Y. Tan and D. W. Agar, Chem. Eng. Technol., 2011, 34, 1481–1489 CrossRef CAS.
- I. M. Al-Najjar and M. Green, J. Chem. Soc., Dalton Trans., 1979, 1651–1656 RSC.
- C. W. Hoerr, M. R. McCorkle and A. W. Ralston, J. Am. Chem. Soc., 1943, 65, 328–329 CrossRef CAS.
- Toxicity Estimation Software Tool, version 4.1, U.S. Environmental Protection Agency, http://www.epa.gov/nrmrl/std/qsar/qsar.html (accessed 2 August, 2013) Search PubMed.
- Y. Takeuchi, Environ. Res., 1993, 62, 76–80 CrossRef CAS PubMed.
- O. F. Sendur, Y. Turan, S. Bal and A. Gurgan, Inhalation Toxicol., 2009, 21, 210–214 CrossRef CAS PubMed.
- G. D. Ritchie, K. R. Still, W. K. Alexander, A. F. Nordholm, C. L. Wilsom, J. Rossi III and D. R. Mattie, J. Toxicol. Environ. Health, Part B, 2001, 4, 223–312 CAS.
- Sax's Dangerous Properties of Industrial Materials, ed. R. J. Lewis, Van Nostrand Reinhold, New York, 9th edn, 1996 Search PubMed.
- E. J. Gallegos, Anal. Chem., 1981, 53, 187–189 CrossRef CAS.
- G. G. Hawley, The Condensed Chemical Dictionary, Van Nstrand Reinhold, New York, 9th edn, 1977 Search PubMed.
- Material safety data sheet for N,N-dimethylcyclohexylamine, Sigma Aldrich, 2012.
- Material safety data sheet for N-ethylpiperidine, Merck Millipore, 2010.
- W. B. Wright Jr., J. Org. Chem., 1962, 27, 1042–1045 CrossRef.
- H. Yoshikawa and K. Kawai, Ind. Health, 1966, 4, 63 CrossRef CAS.
- Material safety data sheet for N-butylpyrrolidine, Sigma Aldrich, 2012.
- T. Caronna, A. Citterio, T. Crolla, M. Ghirardini and F. Minisci, J. Heterocycl. Chem., 1976, 13, 955–960 CrossRef CAS.
- Material safety data sheet for N,N-dimethylhexylamine, Sigma Aldrich, 2012.
- Material safety data sheet for N,N-dimethylbutylamine, Oxea Chemicals, 2011.
- W. H. Bell, G. B. Carter and J. Dewing, J. Chem. Soc. C, 1969, 352–354 RSC.
- A. R. Lepley and W. A. Khan, J. Org. Chem., 1966, 31, 2061–2064 CrossRef CAS.
- Material safety data sheet for N,N-diethylbutylamine, Sigma Aldrich, 2012.
- N. C. Deno and R. E. Fruit, J. Am. Chem. Soc., 1968, 90, 3502–3506 CrossRef CAS.
- Material safety data sheet for N-methyldipropylamine, Sigma Aldrich, 2010.
- G. R. Colonna and A. B. Spencer, Fire Protection Guide to Hazardous Materials, National Fire Protection Association, Quincy, Massachusetts, 13th edn, 2002 Search PubMed.
- Material safety data sheet for triethylamine, Sigma Aldrich, 2012.
- R. E. Watson, A. M. Hafez, J. N. Kremsky and G. O. Bizzigotti, Int. J. Toxicol., 2007, 26, 503–512 CrossRef CAS PubMed.
- P. A. A. Klusener, L. Tip and L. Brandsma, Tetrahedron, 1991, 47, 2041–2064 CrossRef CAS.
- Material safety data sheet for diisopropylaminoethanol, Sigma Aldrich, 2012.
- W. B. Burnett, R. L. Jenkins, C. H. Peet, E. E. Dreger and R. Adams, J. Am. Chem. Soc., 1937, 59, 2248–2252 CrossRef CAS.
- Material safety data sheet for dibutylaminoethanol, Sigma Aldrich, 2013.
- P. R. Muddasani and V. C. Nannapaneni, PCT IntlWO03101931, 2003 Search PubMed.
- Material safety data sheet for 4,4-diethoxy-N,N-dimethyl butan-1-amine, Sigma Aldrich, 2012.
- G. W. Gribble and C. F. Nutaitis, Synthesis, 1987, 709–711 CrossRef CAS.
- Material safety data sheet for N,N-dimethylphenethylamine, Sigma Aldrich, 2013.
- P. G. Stone and S. G. Cohen, J. Am. Chem. Soc., 1982, 104, 3435–3440 CrossRef CAS.
- Material safety data sheet for dipropylamine, Sigma Aldrich, 2012.
- F. Franks and B. Watson, Trans. Faraday Soc., 1967, 63, 329–334 RSC.
- Material safety data sheet for di-sec-butylamine, Sigma Aldrich, 2012.
- D. G. Norton, V. E. Haury, F. C. Davis, L. J. Mitchell and S. A. Ballard, J. Org. Chem., 1954, 19, 1054–1066 CrossRef.
- Material safety data sheet for N-sec-butylpropylamine, TCI Europe, 2012.
- D. Redmore, J. Org. Chem., 1978, 43, 992–996 CrossRef.
- Material safety data sheet for N,N-dimethylbenzylamine, Sigma Aldrich, 2012.
- IUCLID Dataset for Toluene, European Commission – European Chemicals Bureau, 2000 Search PubMed.
- V. A. Benignus, Neurotoxicology, 1981, 2, 567–588 CAS.
- E. T. Kimura, D. M. Ebert and P. W. Dodge, Toxicol. Appl. Pharmacol., 1971, 19, 699–704 CrossRef CAS.
- G. W. Kabalka and S. T. Summers, J. Org. Chem., 1981, 46, 1218–1221 CrossRef.
- Material safety data sheet for toluene, Merck Millipore, 2012.
- N. Chang, Z.-Y. Gu and X.-P. Yan, J. Am. Chem. Soc., 2010, 132, 13645–13647 CrossRef CAS PubMed.
- Material safety data sheet for hexane, Sigma Aldrich, 2012.
- Documentation of the Threshold Limit Values of Biological Exposure Indices, American Conference of Governmental Industrial Hygienists, Cincinnati, Ohio, 6th edn, 1991 Search PubMed.
- Ethel Browning's Toxicity and Metabolism of Industrial Solvents, ed. R. Snyder, Elsevier, New York, 2nd edn, 1987 Search PubMed.
- A. N. Campbell, Can. J. Chem., 1981, 59, 127–131 CrossRef CAS.
- L. M. Besley and G. A. Bottomley, J. Chem. Thermodyn., 1974, 6, 577–580 CrossRef CAS.
- S. A. Wieczorek and J. Stecki, J. Chem. Thermodyn., 1978, 10, 177–186 CrossRef CAS.
- Globally Harmonized System of Classification and Labelling of Chemicals (GHS), United Nations, New York and Geneva, 4th edn, 2011 Search PubMed.
- R. Heijungs, J. Guinee, G. Huppes, R. M. Lankreijer, H. A. Udo de Haes, A. Wegener Sleeswijk, A. M. M. Ansems, P. G. Eggels, R. van Duin and H. P. de Goede, Environmental Life Cycle Assessment of Products. Guide and Backgrounds, CML, Leiden University, Leiden, 1992 Search PubMed.
- J. R. Baker, M. W. Milke and J. R. Mihelcic, Water Res., 1999, 33, 327–334 CrossRef CAS.
- D. T. Allen and D. R. Shonnard, Green Engineering: Environmentally Conscious Design of Chemical Processes, Prentice-Hall, Upper Saddle River, NJ, 2001 Search PubMed.
- W. P. L. Carter, J. Air Waste Manage. Assoc., 1994, 44, 881–899 CAS.
- R. S. Boethling, E. Sommer and D. DiForge, Chem. Rev., 2007, 107, 2207–2227 CrossRef CAS PubMed.
- P. H. Howard, R. S. Boethling, W. M. Stiteler, W. M. Meylan, A. E. Hueber, J. A. Beauman and M. E. Larosche, Environ. Toxicol. Chem., 1992, 11, 593–603 CrossRef CAS.
- I. Eide-Haugmo, O. G. Brakstad, K. A. Hoff, K. R. Sorheim, E. F. da Silva and H. F. Svendsen, Energy Procedia, 2009, 1, 1297–1304 CrossRef CAS PubMed.
- I. Eide-Haugmo, H. Lepaumier, A. Einbu, K. Vernstad, E. F. da Silva and H. F. Svendsen, Energy Procedia, 2011, 4, 1631–1636 CrossRef CAS PubMed.
- I. Eide-Haugmo, O. G. Brakstad, K. A. Hoff, E. F. da Silva and H. F. Svendsen, Int. J. Greenhouse Gas Control, 2012, 9, 184–192 CrossRef CAS PubMed.
- D. Calamari, R. Da Gasso, S. Galassi, A. Provini and M. Vighi, Chemosphere, 1980, 9, 753–762 CrossRef CAS.
- M. Kawasaki, Ecotoxicol. Environ. Saf., 1980, 4, 444–454 CrossRef CAS.
- K. Kawahara, Y. Yakabe, T. Ohide and K. Kida, Chemosphere, 1999, 39, 2007–2018 CrossRef CAS.
- IUCLID Dataset for Cyclohexyldimethylamine, European Commission – European Chemicals Bureau, 2000 Search PubMed.
- E. M. Davis, H. E. Murray, J. G. Liehr and E. L. Powers, Water Res., 1981, 15, 1125–1127 CrossRef CAS.
- G. Pasteris, D. Werner, K. Kaufmann and P. Höhener, Environ. Sci. Technol., 2002, 36, 30–39 CrossRef CAS.
- J. A. Gowland and G. H. Schmid, Can. J. Chem., 1969, 47, 2953–2958 CrossRef CAS.
- B. Zou and H.-F. Jiang, Chin. J. Chem., 2008, 26, 1309–1314 CrossRef CAS.
- S. R. Cheruku, S. Maiti, A. Dorn, B. Scorneaux, A. K. Bhattacharjee, W. Y. Ellis and J. L. Vennerstrom, J. Med. Chem., 2003, 46, 3166–3169 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 13C {1H} NMR spectra of secondary amine and carbonated water mixtures and analysis of the accuracy of TEST predictions. CCDC 967314. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3gc42164c |
| This journal is © The Royal Society of Chemistry 2014 |