Bridging racemic lactate esters with stereoselective polylactic acid using commercial lipase catalysis†
Abstract
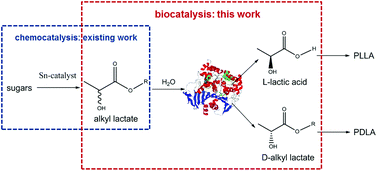
A productive and enantioselective hydrolysis of racemic mixtures of lactate esters with commercial Candida rugosa lipase was performed. This step contributes to a novel envisioned route for stereoselective PLA production by combining recent chemocatalytic developments with this biocatalytic contribution, foreseeing two separate L- and D-lactate enantiomer streams. A study of the hydrolysis kinetics identified an unexpected rate determining step at the origin of an unprecedented ester reactivity order.

 Please wait while we load your content...
Please wait while we load your content...