Pharmaceutical Green Chemistry process changes – how long does it take to obtain regulatory approval?
Abstract
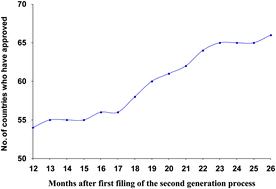
This perspective examines the environmental impact which is the result of variable global approval times in the pharmaceutical industry. In the first part of the paper several case histories are presented demonstrating the positive environmental benefits of second generation chemistry. This positive benefit is partially negated by the variable approval times from regulatory agencies and in the second part of the paper the results of a survey of approval times in the US, EU and globally are presented.
- This article is part of the themed collection: 15 Years of Green Chemistry

 Please wait while we load your content...
Please wait while we load your content...