 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
On the solubility of wood in non-derivatising ionic liquids†
Lasse
Kyllönen
a,
Arno
Parviainen
b,
Somdatta
Deb
b,
Martin
Lawoko
c,
Mikhail
Gorlov
c,
Ilkka
Kilpeläinen
*b and
Alistair W. T.
King
*b
aKemira Oyj, Luoteisrinne 2, P.O. Box 44, Fi-02271 Espoo, Finland. Tel: +358 504099259
bDepartment of Chemistry, University of Helsinki, FIN-00014, P.O. Box 55 (A. I. Virtasen Aukio 1), Helsinki, Finland. E-mail: alistair.king@helsinki.fi; ilkka.kilpelainen@helsinki.fi; Tel: +358 50527 9446
cWallenberg Wood Science Center (WWSC), Department of Fiber and Polymer Technology, Royal Institute of Technology (KTH), Stockholm, Sweden. Fax: +46 8790 8066; Tel: +46 8790 8047
First published on 11th July 2013
Abstract
Norway spruce wood was mechanically pulverized to varying degrees. The solubility of the wood samples, in a range of common ionic and molecular solvents, was quantified using a novel 31P NMR technique. The results show that intact wood is not soluble under mild treatment conditions, in cellulose-dissolving or swelling solvents.
In the future, wood is destined to be the major renewable feedstock for the production of fine chemicals, speciality chemicals, commodity chemicals, materials and fuels. Therefore, efficient solvents for the processing of woody material are in demand. Ionic liquids, as molten salts that melt below 100 °C, appear to be one promising solution. There are however several initial conflicting reports describing the outcome of contacting wood with ionic liquids.1 Different effects or phenomena can be observed in each report. One report describes complete dissolution followed by homogeneous chemical modification.1a Another describes partial dissolution (extraction).1b One report describes wood liquefaction.1d Yet another describes swelling of wood1e and still others describe fibrillation1g or disintegration1f of wood, upon treatment with common cellulose-dissolving ionic liquids.2 Lastly one recent article describes the very efficient fractionation of lignin from polysaccharides.1h In addition to this there are many ensuing reports describing fractionation, resulting from complete dissolution or partial extraction from highly effective cellulose solvents, such as 1-allyl-3-methylimidazolium chloride ([amim]Cl), 2-ethyl-3-methylimidazolium dimethylphosphate ([emim][Me2PO4]) and 1-ethyl-3-methylimidazolium acetate ([emim][OAc]). There is however a general misunderstanding that if a medium is good at dissolving cellulose, it therefore should be capable of dissolving wood, with cellulose as the major constituent. This is a critical mistake as wood is a much more complex substrate than low molecular weight purified cellulose. A recent review by Brandt et al.1i nicely illustrates the complexity of wood as a substrate. The recalcitrance of wood is normally attributed to the insolubility of cellulose in most non-derivatising media. Other factors must also be taken into account, such as the presence of lignin–carbohydrate complexes (LCCs) and the ultrastructure of wood itself. Discerning the solubility of wood in ionic liquids is therefore crucial for process understanding and development.
Previously it has been determined that pulverized (planetary-milled) Norway spruce sawdust is not fully soluble in [amim]Cl under mild dissolution conditions.1c As the aim of this study was to assess the solubility of wood in non-derivatising solvents, we decided to look a little further at a short range of ionic liquids and molecular solvents, or electrolyte systems: [amim]Cl, [emim][Me2PO4], 1-ethyl-3-methylimidazolium trifluoromethanesulphonate ([emim][OTf]), 1-ethyl-3-methylimidazolium bis(trifluoromethanesulphonyl)imide ([emim][NTf2]), d-chloroform (CDCl3), N,N-dimethylacetamide (DMA) and LiCl/DMA. The same wood preparation method was used as in the original publication.1c Kamlet–Taft parameters3a were measured (ESI†)3b or taken from the literature3a for these solvents (Table 1). Three of these media are known to be good solvents for cellulose, as a major component of wood, typically insoluble in most molecular solvent systems: [amim]Cl,2b [emim][Me2PO4]2c and LiCl/DMA.4 This dissolution capability is attributable to their basicity.4,2c,5 DMA was chosen as a polar aprotic solvent, known to partially swell cellulose, but not dissolve it completely. [emim][OTf], [emim][NTf2] and CDCl3 were chosen as non-basic and non-swelling solvents.
[emim][OAc] is commonly quoted as a better solvent than others for wood dissolution. This may be related to the more basic nature of [emim][OAc], in comparison to other stable imidazolium-based ionic liquids.6 In this study it was not possible to compare the dissolution capability of [emim][OAc] to other ionic liquids due to the consumption of the phosphitylating reagent upon introduction into [emim][OAc] solutions. Attempts were made to analyse the same wood samples with [emim][OAc] by varying reaction conditions after introduction of the phosphitylating reagent. These all failed as the acetate forms an unreactive mixed anhydride upon reacting with the reagent.
Reaction of the phosphitylating reagent with [emim][Me2PO4] also results in the formation of a mixed anhydride but dialkylphosphates are rather good leaving groups and this reactivity does not seem to interfere with the phosphitylation of wood from this ionic liquid. In addition, 1-ethyl-3-methylimidazolium ethylsulfate ([emim][EtSO4]) was chosen for the procedure, as Brandt et al.1h have published the use of similar 1-butyl-3-methylimidazolium methylsulfate/sulphate–water mixtures as highly effective media for the fractionation of wood. In this publication there are some questions as to what the mechanism of fractionation actually involves, as there is some speculation that the enhanced acidity of the media may have a catalytic effect, allowing for partial degradation and release of amorphous polysaccharides and lignin.
For the purpose of comparing the solubility of wood in these systems, wood samples were pulverized by planetary milling, according to a previous article.1c Dissolution of these samples into [amim]Cl under mild conditions (80 °C, 18 h) had previously shown that it was only after 48 h of planetary milling, with that specific protocol, that the wood turned into a fully dissolved (homogeneous phase) state. The typical procedure was as follows (Fig. 1): (1) pre-swelling/‘dissolution’ of wood in [amim]Cl, (2) phosphitylation of available hydroxyls, (3) quantitative 31P NMR analysis conditions, and (4) integration against an internal standard. The procedure was thoroughly validated using microcrystalline cellulose in a previous publication7 and is only specific for polymeric phosphite esters in the solution-state, i.e. not resonances arising from swollen or gel-state particles.
 | ||
| Fig. 1 31P NMR analysis of dried and planetary-milled spruce (Picea abies) heartwood, after swelling/solvation with ionic liquid or molecular solvent (90 °C, 18 h), phosphitylation of hydroxyls (pyridine and phosphitylating reagent) and quantification of solution-state phosphite esters, as a measure of wood solubility. | ||
31P NMR analysis was run according to an optimised procedure (see ESI†) although dissolution was performed at 90 °C for a maximum of 18 h. This is a 10 °C rise from the previous procedure although not enough to cause significant degradation of the polymer matrix. The results from this analysis (‘total available hydroxyls’, as a function of milling time) are given in Fig. 1. The concept of wood ‘solubility’, as assessed using this procedure, is rather abstract. The quantification is representative of the ‘available hydroxyls in solution’, which arise from dissolution of low-enough molecular weight fractions. Likely the bulk of the hydroxyls in wood are esterified using this highly electrophilic reagent. However, it is only those ester moieties which are mobile in the isotropic liquid that are quantifiable by the NMR procedure. Therefore, in essence we are measuring the degree of accessibility of hydroxyls, in the swollen state, and not the traditional concept of ‘solubility’, as such.
All of the systems, except CDCl3, [emim][EtSO2], [emim][OTf] and [emim][NTf2], were capable of giving total hydroxyl values close to that of [amim]Cl for all milled samples. It is not unexpected for these media not to swell or dissolve wood. Their basicities, as parameterised by the Kamlet–Taft β value (Table 1), are the lowest of the series. Brandt et al.1e also reported that 1-butyl-3-methylimidazolium triflate ([bmim][OTf]), structurally very similar to [emim][OTf], was very poor at swelling wood. It has a β value above that of CDCl3 and [emim][NTf2] but not higher than DMA (only capable of swelling cellulose). On the opposite end of the scale, it is not unexpected for [amim]Cl, LiCl/DMA and [emim][Me2PO4] to be good solvents for wood, based on their abilities to dissolve cellulose. It is interesting to note there that both DMA and [emim][OTf] do not dissolve cellulose, yet after about ∼50 h milling time they both give total hydroxyl values close to the values obtained from pre-solvation in [amim]Cl. It appears that even though these solvents do not dissolve cellulose or swell wood, there is a degree of reactive dissolution, for the pulverized samples, brought on by partial swelling of the wood matrix. This is likely due to swelling of the lignin in the complex lignocellulosic matrix as [emim][OTf] and DMA are known to be very effective solvents for lignin but CDCl3 and [emim][NTf2] are very poor solvents for both lignin and polysaccharides. When [emim][EtSO4] was analysed, all the spectra showed the formation of low molecular weight resonances (example in Fig. 2). This is a strong indication that degradation occurs during the ionic liquid treatment and may be an indication as to why this class of ionic liquids is so successful at liberating the lignin and hemicellulose from the cellulosic crystallites in the substrate. Water is however absent in this analysis procedure, which may have a stabilising effect during the fractionation procedure. If amorphous polysaccharides (hemicelluloses and amorphous regions of cellulose) are being digested in such a fashion, this could be confirmed by the appearance of nanostructured cellulose, e.g. cellulose nanocrystals (CNCs), using AFM or similar visualisation techniques. The formation of CNCs has already been confirmed with pure 1-butyl-3-methyl hydrogensulfate ([bmim][HSO4]) during the treatment of microcrystalline cellulose (MCC).8 This is the same ionic liquid that is used in the Brandt publication for fractionation.
![31P NMR analysis of Norway spruce, planetary milled for 96 h and pre-dissolved in [emim][EtSO4]. Sharp resonances indicate significant degradation of polysaccharides.](/image/article/2013/GC/c3gc41273c/c3gc41273c-f2.gif) | ||
| Fig. 2 31P NMR analysis of Norway spruce, planetary milled for 96 h and pre-dissolved in [emim][EtSO4]. Sharp resonances indicate significant degradation of polysaccharides. | ||
The overriding conclusion however is that none of the media tested in this study were able to dissolve spruce wood sawdust until in a highly pulverized state (>50 h planetary-milling using this procedure). Moreover, samples of Wiley-milled (40-mesh) Norway spruce (Picea abies, a representative softwood), birch (Betula pendula, a representative hardwood) and corn stover (Zea mays, a representative grass-based feedstock) were also prepared and analysed using the same procedure ([amim]Cl dissolution). None of the wood substrates dissolved to a significant extent, in comparison to the 96 h planetary-milled spruce sawdust (Fig. 3). The values for the Wiley-milled materials were even slightly lower than those for the spruce sawdust, attributable to their slightly larger particle size. The corn stover sample however dissolved to a higher degree but not completely, in comparison to the 96 h planetary milled wood. Therefore, it can be stated that wood is only partially soluble in non-derivatising ionic liquids, or any other direct dissolution media for that matter. It is only after extended pulverization that the material becomes soluble. This clearly is a result of fragmentation of the insoluble lignocellulosic matrix and may be linked to the presence of LCCs.9
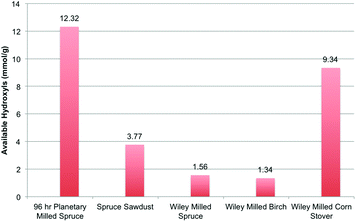 | ||
| Fig. 3 31P NMR analysis of Norway spruce, planetary milled for 96 h, spruce sawdust, and Wiley-milled spruce, birch and corn stover. | ||
Factors such as cellulose crystallinity and ultrastructure effects, affecting solvent diffusion through the cell wall and mass transport of bulk polymer, clearly will also play a role. One could argue however that ultrastructure effects are merely a combination of the LCC (covalent) linkages between high molecular weight polymers into an ‘extended LCC matrix’. Nevertheless, assessing and rationalising these contributions to wood solubility, fractionation mechanisms or ‘recalcitrance reduction’ must be the focus of future work to improve our understanding of wood solubility and processing. The implications of this insolubility, for wood fractionation, are very important. Due to the haphazard way in which authors report complete dissolution of wood, prior to selective fractionation, there is a lot of misinformation and misunderstanding in the literature surrounding this topic. This total dissolution/precipitation concept is distinct from extraction, which is the more likely explanation for most reports although the variation between the two obviously depends on the degree of degradation, arising from pre-treatments, media reactivity or impurities in the dissolution media. [emim][OAc] is commonly quoted to give better solubility of wood, although this ionic liquid is known to be a derivatising ionic liquid and this reactivity6 may allow for a ‘reactive dissolution’. An article by Sun et al.10a describes the almost complete solubility of wood, of varying particle sizes, in [emim][OAc]. Greater than 90% of the material was soluble, as opposed to around 50% and lower for 1-butyl-3-methylimidazolium chloride ([bmim]Cl). Xie et al.10b have also demonstrated a similar effect on the dissolution of corn stover into [emim][OAc]-NMP vs. [bmim]Cl- and [amim]Cl-NMP electrolytes. The authors follow the state of dissolution by using a novel fluorescence microscopy technique whereby lignin disperses into the media only after fragmentation of the cell-wall crystallites. It appears that [emim][OAc] has a distinct advantage when it comes to dissolution, most likely attributable to the increased anion basicity, allowing for further chemistry. [emim][OAc] is already known to interact covalently with woody biomass, through cellulose reducing end conjugation11a,b and transacetylation.11c,d Carbenes, which are shown even to be present at room temperature in the pure [emim][OAc],12 may be key to this reactivity. None of this reactivity has been reported for the chloride or dialkylphosphate series of ionic liquids. If this chemistry is already occurring with carbonyl functionalities in wood, then cleavage of labile LCCs is also expected. Articles by Leskinen et al.9 demonstrate an ability to extract cellulose from the insoluble Norway spruce LCC matrix, using [amim]Cl. As we have not observed the chloride series to induce this chemistry, under mild dissolution conditions, and it is understood that cellulose is not linked to lignin, to any significant extent in Norway spruce, cellulose extraction seems logical. Although no direct evidence of LC bonds in native wood has been provided, there is general consensus of their existence based on indirect chemical analyses.13a Quantitative isolation methods have revealed that all lignin in spruce exists as an LCC matrix, where lignin forms networks with hemicellulose, but not with cellulose.13b Obviously, such networks would impede dissolution of the matrix unless effectively broken. It must be mentioned that, while important studies are being carried out into the NMR quantification of LCCs in isolated fractions,13c assignments are based on model compounds and the abundances of resonances are too low to allow for full assignment of the spin systems of the linking monomers, by multidimensional NMR. This is significant as there is still a large proportion of lignin structure that is unknown. Therefore, for some, the existence of LCCs in native wood is often disputed, despite the mass of experimental evidence.13 In this regard, it is asserted that indirect chemical analysis, which frequently requires extensive pulverisation and chemical isolation, may introduce LCC artefacts. The goals of this area of research are long term and the benefits are very significant. In terms of ionic liquid reactivity towards LCCs, if the increased basicity of the acetate series is allowing for chemistry to occur, through direct covalent interaction of acetate or via a C2-carbene intermediate, this is likely a catalytic effect and should not affect the sustainability of processes, through consumption of the ionic liquid. This clearly needs further proof and has strong implications for both understanding fractionation mechanisms and process techno-economics.
Other factors affecting the dissolution capability of ionic liquids in general are contaminating impurities (e.g. acid) that can catalyse the wholesale degradation of wood biopolymers and, as such, allow for rapid and complete solubilisation. Higher temperature ranges14 clearly also cause significant degradation of wood biopolymers, aiding solubility. While wood is dissolving under these conditions, this is a reactive dissolution and, as such, should be distinct from non-degradative dissolution, in terms of rationalising the process mechanisms and efficiencies. In this regard there is no evidence that the glass-transition of lignin14a has any effect on the dissolution of wood, as opposed to chemistry that is occurring close to the decomposition point of the ionic liquid.6,14a It must be said, however, that this degradation is somewhat desirable for biofuel-related pre-treatments, provided the recovery of relatively pure ionic liquids is very high. One should balance the kinetics of ionic liquid decomposition vs. biopolymer decomposition under different conditions.
If the preservation of crystallinity, molecular weight or fibrous properties is preferred for particular product specifications (e.g. nanocellulose, dissolving pulps or paper pulps respectively), then selective LCC cleavage, partial LCC matrix fragmentation or degradation of only the amorphous polysaccharides would be preferable, depending on the pulp specifications. There are already indications of pre-treatments that can achieve this. For example mild aqueous acid/base treatments or autohydrolysis15 are known to drastically increase the solubility of intact wood, while maintaining quite high yields from the initial untreated material. Additional hemicellulose-rich fractions from hot water extraction could also add significant value to a process. These selective pre-treatments may open the door to full and more efficient homogeneous processing of wood in non-derivatising ionic liquids. Autohydrolysis is of particular importance as it is already used in the production of pre-hydrolysis kraft pulp and more acidic or basic pre-treatments tend to induce further structural changes.
With regard to chemical modification of wood, basic ionic liquids or molecular solvents, such as those described here, may not be necessary for efficient modification of the substrate. This is demonstrated by the ability of DMA and [emim][OTf] to allow for full solubilisation of the 96 h planetary-milled wood, despite the inability of these solvents to dissolve cellulose. This is likely due to swelling of the lignin-rich portions of the fragmented LCC matrix, allowing for a reactive dissolution. There will still be the solubility limit however, unless further chemical or mechanical methods are applied to improve the initial solubility. From a technical point of view, Norway spruce wood contains ∼12 mmol of aliphatic hydroxyls per gram of wood. This should be the starting point for the atom efficient modification of wood, to produce sustainable materials and chemicals.
Notes and references
- (a) I. Kilpeläinen, H. Xie, A. W. T. King, M. Granström, S. Heikkinen and D. S. Argyropoulos, J. Agric. Food Chem., 2007, 55, 9142 CrossRef; (b) D. A. Fort, R. C. Remsing, R. P. Swatloski, P. Moyna, G. Moyna and R. D. Rogers, Green Chem., 2007, 9, 63 RSC; (c) A. W. T. King, L. Zoia, I. Filpponen, A. Olszewska, H. Xie, I. Kilpeläinen and D. S. Argyropoulos, J. Agric. Food Chem., 2009, 57, 8236 CrossRef CAS; (d) H. Xie and T. Shi, Holzforschung, 2006, 60, 509 Search PubMed; (e) A. Brandt, J. P. Hallett, D. J. Leak, R. J. Murphy and T. Welton, Green Chem., 2010, 12, 672 RSC; (f) J. Viell and W. Marquardt, Holzforschung, 2011, 65, 519 CrossRef CAS; (g) I. Kilpeläinen, A. W. T. King, P. Karhunen and J. Matikainen, Patent, WO 2011/114004 A1 Search PubMed; (h) A. Brandt, M. J. Ray, T. Q. To, D. J. Leak, R. J. Murphy and T. Welton, Green Chem., 2011, 13, 2489 RSC; (i) A. Brandt, J. Gräsvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550 RSC.
- (a) R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974 CrossRef CAS; (b) H. Zhang, J. Wu, J. Zhang and J. S. He, Macromol., 2005, 38, 8272 CrossRef CAS; (c) Y. Fukaya, K. Hayashi, M. Wada and H. Ohno, Green Chem., 2008, 10, 44 RSC; (d) Y. Fukaya, A. Sugimoto and H. Ohno, Biomacromolecules, 2006, 7, 3295 CrossRef CAS.
- (a) M. J. Kamlet, J. L. M. Abboud, M. H. Abraham and R. W. Taft, J. Org. Chem., 1983, 48, 2877 CrossRef CAS; (b) A. Parviainen, A. W. T. King, I. Mutikainen, H. Hummel, C. Selg, L. K. J. Hauru, H. Sixta and I. Kilpeläinen, ChemSusChem, 2013 DOI:10.1002/cssc.201300143.
- S. Spange, A. Reuter, E. Vilsmeier, T. Heinze, D. Keutel and W. Linert, Polym. Chem., 1998, 36, 1945 CrossRef CAS.
- L. K. J. Hauru, M. Hummel, A. W. T. King, I. Kilpeläinen and H. Sixta, Biomacromolecules, 2012, 13, 2896 CrossRef CAS.
- A. W. T. King, A. Parviainen, P. Karhunen, J. Matikainen, L. K. J. Hauru, H. Sixta and I. Kilpeläinen, RSC Adv., 2012, 2, 8020 RSC.
- A. W. T. King, I. Kilpeläinen, S. Heikkinen, P. Järvi and D. S. Argyropoulos, Biomacromolecules, 2009, 10, 458 CrossRef CAS.
- Z. Man, N. Muhammad, A. Sarwono, M. Azmi Bustam, M. Vignesh Kumar and S. Rafiq, J. Polym. Environ., 2011, 19, 726 CrossRef CAS.
- (a) T. Leskinen, A. W. T. King, I. Kilpeläinen and D. S. Argyropoulos, Ind. Eng. Chem. Res., 2011, 50, 12349 CrossRef CAS; (b) T. Leskinen, A. W. T. King, I. Kilpeläinen and D. S. Argyropoulos, Ind. Eng. Chem. Res., 2013, 52, 3958 CrossRef CAS.
- (a) N. Sun, M. Rahman, Y. Qin, M. L. Maxim, H. Rodríguez and R. D. Rogers, Green Chem., 2009, 11, 646 RSC; (b) H. Xie, H. Shen, Z. Gong, Q. Wang, Z. K. Zhao and F. Bai, Green Chem., 2012, 14, 1202 RSC.
- (a) T. Liebert and T. Heinze, Bioresources, 2008, 3, 576 Search PubMed; (b) G. Ebner, S. Schiehser, A. Potthast and T. Rosenau, Tetrahedron Lett., 2008, 49, 7322 CrossRef CAS; (c) Ö. P. Çetinkol, D. C. Dibble, G. Cheng, M. S. Kent, B. Knierim, M. Auer, D. E. Wemmer, J. G. Pelton, Y. B. Melnichenko, J. Ralph, B. A. Simmons and B. M. Holmes, Biofuels, 2010, 1, 33 CrossRef; (d) L. K. J. Hauru, M. Hummel and H. Sixta, Books of Abstracts, 241st ACS National Meeting, Anaheim, CA, March 27–31, 2011, American Chemical Society, Washington, DC, 2011, CELL-297 Search PubMed.
- (a) R. Rodríguez, G. Gurau, J. D. Holbrey and R. D. Rogers, Chem. Commun., 2011, 47, 3222 RSC; (b) G. Gurau, R. Rodríguez, J. Kelley, P. Janiczek, R. S. Kalb and R. D. Rogers, Angew. Chem., Int. Ed., 2011, 47, 3222 Search PubMed.
- (a) T. Koshijima and T. Watanabe, Association between Lignin and Carbohydrates in Wood and Other Plant Tissues, Springer, Heidelberg, 2003 Search PubMed; (b) M. Lawoko, G. Henriksson and G. Gellerstedt, Biomacromolecules, 2005, 6, 3467 CrossRef CAS; (c) M. Balakshin, E. Capanema, H. Gracz, H. M. Chang and H. Jameel, Planta, 2011, 233, 1097 CrossRef CAS.
- (a) W. Li, N. Sun, B. Stoner, X. Jiang, X. Lu and R. D. Rogers, Green Chem., 2011, 13, 2038 RSC; (b) N. Sun, W. Li, B. Stoner, X. Jiang, X. Lu and R. D. Rogers, Green Chem., 2011, 13, 1158 RSC.
- (a) L. K. J. Hauru, Y. Ma, M. Hummel and H. Sixta, Books of Abstracts, 243rd ACS National Meeting, San Diego, CA, March 25–29, 2012, American Chemical Society, Washington, DC, 2012, CELL-93 Search PubMed; (b) L. K. J. Hauru, Y. Ma, M. Hummel, M. Alekhina, A. W. T. King, I. Kilpeläinen, P. A. Penttilä, R. Serimaa and H. Sixta, RSC Adv., 2013 10.1039/C3RA41529E.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the ionic liquid preparation, the wood sample preparation, the 31P NMR procedure and the Kamlet–Taft procedure are given here. See DOI: 10.1039/c3gc41273c |
| This journal is © The Royal Society of Chemistry 2013 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)